Adaptive responses of murine osteoblasts subjected to coupled mechanical stimuli
- PMID: 28957700
- PMCID: PMC5696036
- DOI: 10.1016/j.jmbbm.2017.09.018
Adaptive responses of murine osteoblasts subjected to coupled mechanical stimuli
Abstract
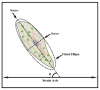
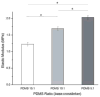
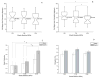
Restitution of the natural organization and orientation of cells is imperative for the construction of functional tissue scaffolds. While numerous studies have exploited mechanical methods to engineer tissues with the desired cellular architecture, fundamental knowledge is still lacking in understanding the manner in which morphological features can be modulated through coupled mechanical cues. To address this knowledge gap, the adhesion and alignment response of murine osteoblast cells under the synergistic effects of matrix rigidity and cyclic mechanical loading was investigated. This was accomplished by applying cyclic mechanical strain (1% at 0.05Hz) to MC3T3-E1 cells seeded on PDMS substrates of different elastic moduli (1.22, 1.70 and 2.04MPa). Results demonstrate that the overall cell density and expression of inactive vinculin increased on substrates subjected to cyclic stimulus in comparison to substrates under static loading. Conversely, in terms of the adhesion response, osteoblasts exhibited an increased growth of focal adhesion complexes under static substrates. Interestingly, results also elucidate that substrates of a stiffer matrix exposed to cyclic stimulus, had a significantly higher percentage of osteoblasts aligned parallel to the direction of the applied strain, as well as a higher degree of internal order with respect to the strain axis, in comparison to both cells seeded on substrates of lower stiffness under cyclic loading or under static conditions. These findings suggest the role of cyclic mechanical strain coupled with matrix rigidity in eliciting mechanosensitive adaptations in cell functions that allow for the reconstitution of the spatial and orientational assembly of cells in vivo for tissue engineering.
Keywords: Cell orientation; Cyclic load; MC3T3-E1 cells; Mechanical stimulus; Vinculin cloud.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors have a conflict in interest in carrying out or based on the results in this study. The study has been carried out under the strict guidelines of research ethics and integrity.
Figures


References
-
- Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nature Methods. 2006;3:369–375. - PubMed
-
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. European Journal of Cell Biology. 2006;85:165–173. - PubMed
-
- Boada-Lopez J, DeJesus-Maldonado I, Jerez J, Romañach R, Diffoot-Carlo N, Sundaram P. Collagen abundance in mechanically stimulated osteoblast cultures using near infrared microscopy. Journal of Biomechanics. 2013;46:2442–2450. - PubMed
-
- Brunette DM, Chehroudi B. The Effects of the Surface Topography of Micromachined Titanium Substrata on Cell Behavior in Vitro and in Vivo. Journal of Biomechanical Engineering. 1999;121(1):49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

