Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial
- PMID: 28958504
- PMCID: PMC5898242
- DOI: 10.1016/S1470-2045(17)30566-1
Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial
Abstract
Background: Rilotumumab is a fully human monoclonal antibody that selectively targets the ligand of the MET receptor, hepatocyte growth factor (HGF). We aimed to assess the efficacy, safety, and pharmacokinetics of rilotumumab combined with epirubicin, cisplatin, and capecitabine, and to assess potential biomarkers, in patients with advanced MET-positive gastric or gastro-oesophageal junction adenocarcinoma.
Methods: This multicentre, randomised, double-blind, placebo-controlled, phase 3 study was done at 152 centres in 27 countries. We recruited adults (aged ≥18 years) with unresectable locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, MET-positive tumours (≥25% of tumour cells with membrane staining of ≥1+ staining intensity), and evaluable disease, who had not received previous systemic therapy. Eligible patients were randomly assigned (1:1) via a computerised voice response system to receive rilotumumab 15 mg/kg intravenously or placebo in combination with open-label chemotherapy (epirubicin 50 mg/m2 intravenously; cisplatin 60 mg/m2 intravenously; capecitabine 625 mg/m2 orally twice daily) in 21-day cycles for up to ten cycles. After completion of chemotherapy, patients continued to receive rilotumumab or placebo monotherapy until disease progression, intolerability, withdrawal of consent, or study termination. Randomisation was stratified by disease extent and ECOG performance status. Both patients and physicians were masked to study treatment assignment. The primary endpoint was overall survival, analysed by intention to treat. We report the final analysis. This study is registered with ClinicalTrials.gov, number NCT01697072.
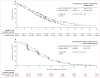
Findings: Between Nov 7, 2012, and Nov 21, 2014, 609 patients were randomly assigned to rilotumumab plus epirubicin, cisplatin, and capecitabine (rilotumumab group; n=304) or placebo plus epirubicin, cisplatin, and capecitabine (placebo group; n=305). Study treatment was stopped early after an independent data monitoring committee found a higher number of deaths in the rilotumumab group than in the placebo group; all patients in the rilotumumab group subsequently discontinued all study treatment. Median follow-up was 7·7 months (IQR 3·6-12·0) for patients in the rilotumumab group and 9·4 months (5·3-13·1) for patients in the placebo group. Median overall survival was 8·8 months (95% CI 7·7-10·2) in the rilotumumab group compared with 10·7 months (9·6-12·4) in the placebo group (stratified hazard ratio 1·34, 95% CI 1·10-1·63; p=0·003). The most common grade 3 or worse adverse events in the rilotumumab and placebo groups were neutropenia (86 [29%] of 298 patients vs 97 [32%] of 299 patients), anaemia (37 [12%] vs 43 [14%]), and fatigue (30 [10%] vs 35 [12%]). The frequency of serious adverse events was similar in the rilotumumab and placebo groups (142 [48%] vs 149 [50%]). More deaths due to adverse events occurred in the rilotumumab group than the placebo group (42 [14%] vs 31 [10%]). In the rilotumumab group, 33 (11%) of 298 patients had fatal adverse events due to disease progression, and nine (3%) had fatal events not due to disease progression. In the placebo group, 23 (8%) of 299 patients had fatal adverse events due to disease progression, and eight (3%) had fatal events not due to disease progression.
Interpretation: Ligand-blocking inhibition of the MET pathway with rilotumumab is not effective in improving clinical outcomes in patients with MET-positive gastric or gastro-oesophageal adenocarcinoma.
Funding: Amgen.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All other authors declare no competing interests.
Figures




Comment in
-
Continued disappointments with targeted agents in first-line therapy of advanced gastric cancers.Lancet Oncol. 2017 Nov;18(11):1427-1428. doi: 10.1016/S1470-2045(17)30714-3. Epub 2017 Sep 25. Lancet Oncol. 2017. PMID: 28958501 No abstract available.
References
-
- WHO International Agency for Research on Cancer. [accessed June 5, 2017];Cancer fact sheets. http://gco.iarc.fr/today/fact-sheets-cancers?cancer=29&type=0&sex=0.
-
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
-
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. - PubMed
-
- Kawakami H, Okamoto I. MET-targeted therapy for gastric cancer: the importance of a biomarker-based strategy. Gastric Cancer. 2016;19:687–95. - PubMed
-
- Drebber U, Baldus SE, Nolden B, et al. The overexpression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep. 2008;19:1477–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

