Guanidinylated Neomycin Conjugation Enhances Intranasal Enzyme Replacement in the Brain
- PMID: 28958576
- PMCID: PMC5768556
- DOI: 10.1016/j.ymthe.2017.08.007
Guanidinylated Neomycin Conjugation Enhances Intranasal Enzyme Replacement in the Brain
Abstract
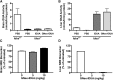
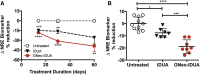
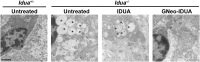
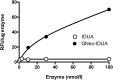
Iduronidase (IDUA)-deficient mice accumulate glycosaminoglycans in cells and tissues and exhibit many of the same neuropathological symptoms of patients suffering from Mucopolysaccharidosis I. Intravenous enzyme-replacement therapy for Mucopolysaccharidosis I ameliorates glycosaminoglycan storage and many of the somatic aspects of the disease but fails to treat neurological symptoms due to poor transport across the blood-brain barrier. In this study, we examined the delivery of IDUA conjugated to guanidinoneomycin (GNeo), a molecular transporter. GNeo-IDUA and IDUA injected intravenously resulted in reduced hepatic glycosaminoglycan accumulation but had no effect in the brain due to fast clearance from the circulation. In contrast, intranasally administered GNeo-IDUA entered the brain rapidly. Repetitive intranasal treatment with GNeo-IDUA reduced glycosaminoglycan storage, lysosome size and number, and neurodegenerative astrogliosis in the olfactory bulb and primary somatosensory cortex, whereas IDUA was less effective. The enhanced efficacy of GNeo-IDUA was not the result of increased nose-to-brain delivery or enzyme stability, but rather due to more efficient uptake into neurons and astrocytes. GNeo conjugation also enhanced glycosaminoglycan clearance by intranasally delivered sulfamidase to the brain of sulfamidase-deficient mice, a model of Mucopolysaccharidosis IIIA. These findings suggest the general utility of the guanidinoglycoside-based delivery system for restoring missing lysosomal enzymes in the brain.
Keywords: enzyme-replacement therapy; guanidinoglycosides; intranasal delivery; mucopolysaccharidoses; neuropathology.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Esko J.D., Selleck S.B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. - PubMed
-
- Neufeld E.F., Muenzer J. The mucopolysaccharidoses. In: Scriver C.R., Sly W.S., Childs B., Beaudet A.L., Valle D., Kinzler K.W., Vogelstein B., editors. Eighth Edition. Volume 3. MacGraw-Hill; 2001. pp. 3421–3452. (Metabolic and Molecular Basis of Inherited Disease).
-
- Kelly J.M., Bradbury A., Martin D.R., Byrne M.E. Emerging therapies for neuropathic lysosomal storage disorders. Prog. Neurobiol. 2017;152:166–180. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

