Impact of rotavirus vaccine on all-cause diarrhea and rotavirus hospitalizations in Madagascar
- PMID: 28958809
- PMCID: PMC5867203
- DOI: 10.1016/j.vaccine.2017.08.091
Impact of rotavirus vaccine on all-cause diarrhea and rotavirus hospitalizations in Madagascar
Abstract
Background: Rotavirus vaccine was introduced into the Extended Program on Immunization in Madagascar in May 2014. We analyzed trends in prevalence of all cause diarrhea and rotavirus hospitalization in children <5years of age before and after vaccine introduction and assessed trend of circulating rotavirus genotypes at Centre Hospitalier Universitaire Mère Enfant Tsaralalàna (CHU MET).
Methods: From January 2010 to December 2016, we reviewed the admission logbook to observe the rate of hospitalization caused by gastroenteritis among 19619 children <5years of age admitted at the hospital. In June 2013-December 2016, active rotavirus surveillance was also conducted at CHUMET with support from WHO. Rotavirus antigen was detected by EIA from stool specimen of children who are eligible for rotavirus gastroenteritis surveillance at sentinel site laboratory and rotavirus positive specimens were further genotyped at Regional Reference Laboratory by RT-PCR.
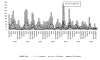
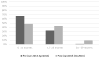
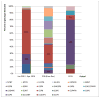
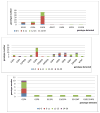
Results: Diarrhea hospitalizations decreased after rotavirus vaccine introduction. The median proportion of annual hospitalizations due to diarrhea was 26% (range: 31-22%) before vaccine introduction; the proportion was 25% the year of vaccine introduction, 17% in 2015 and 16% in 2016. Rotavirus positivity paralleled patterns observed in diarrhea. Before vaccine introduction, 56% of stool specimens tested positive for rotavirus; the percent positive was 13% in 2015, 12% in 2016. Diverse genotypes were detected in the pre-vaccine period; the most common were G3P[8] (n=53; 66%), G2P[4] (n=12; 15%), and G1P[8] (n=11; 14%). 6 distinct genotypes were found in 2015; the most common genotype was G2P[4] (n=10; 67%), the remaining, 5, G12[P8], G3[P8], G1G3[P4], G3G12[P4][P8] and G1G3[NT] had one positive specimen each.
Conclusions: Following rotavirus vaccine introduction all-cause diarrhea and rotavirus-specific hospitalizations declined dramatically. The most common genotypes detected in the pre-vaccine period were G3P[8] and G2P[4] in 2015, the post vaccine period.
Keywords: Genotype; Rotavirus; Rotavirus vaccine; Surveillance.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


References
-
- World Health 0rganization. Weekly epidemiological record No 47. 2008;83:421–4282.
-
- Babji S, Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol. 2012;2:443–448. - PubMed
-
- WHO. Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009;84:533–540. - PubMed
-
- Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;88:49–64. - PubMed
-
- Glass RI, et al. Rotavirus vaccines: Current prospects and challenges. Lancet. 2006;368:323–332. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

