High-throughput RNA structure probing reveals critical folding events during early 60S ribosome assembly in yeast
- PMID: 28959008
- PMCID: PMC5620067
- DOI: 10.1038/s41467-017-00761-8
High-throughput RNA structure probing reveals critical folding events during early 60S ribosome assembly in yeast
Abstract
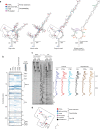
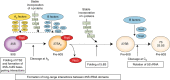
While the protein composition of various yeast 60S ribosomal subunit assembly intermediates has been studied in detail, little is known about ribosomal RNA (rRNA) structural rearrangements that take place during early 60S assembly steps. Using a high-throughput RNA structure probing method, we provide nucleotide resolution insights into rRNA structural rearrangements during nucleolar 60S assembly. Our results suggest that many rRNA-folding steps, such as folding of 5.8S rRNA, occur at a very specific stage of assembly, and propose that downstream nuclear assembly events can only continue once 5.8S folding has been completed. Our maps of nucleotide flexibility enable making predictions about the establishment of protein-rRNA interactions, providing intriguing insights into the temporal order of protein-rRNA as well as long-range inter-domain rRNA interactions. These data argue that many distant domains in the rRNA can assemble simultaneously during early 60S assembly and underscore the enormous complexity of 60S synthesis.Ribosome biogenesis is a dynamic process that involves the ordered assembly of ribosomal proteins and numerous RNA structural rearrangements. Here the authors apply ChemModSeq, a high-throughput RNA structure probing method, to quantitatively measure changes in RNA flexibility during the nucleolar stages of 60S assembly in yeast.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

