Endoplasmic reticulum stress preconditioning modifies intracellular mercury content by upregulating membrane transporters
- PMID: 28959040
- PMCID: PMC5620048
- DOI: 10.1038/s41598-017-09435-3
Endoplasmic reticulum stress preconditioning modifies intracellular mercury content by upregulating membrane transporters
Abstract
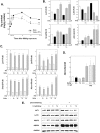
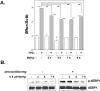
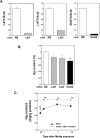
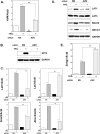
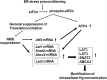
Endoplasmic reticulum (ER) stress preconditioning protects cells against methylmercury (MeHg) cytotoxicity by inducing integrated stress responses such as eIF2α phosphorylation, ATF4 accumulation, and nonsense-mediated mRNA decay (NMD) suppression. Here we demonstrated that ER stress preconditioning results in the upregulation of membrane transporters, leading to a decrease in intracellular mercury content. Our analyses showed that ER stress preconditioning upregulated the expression of methionine transporters that affect the cellular influx of MeHg, LAT1, LAT3, and SNAT2; and a membrane transporter that affects the efflux of MeHg, ABCC4, in MeHg-susceptible myogenic cells. Among these, ABCC4 transporter expression exhibited the greatest elevation. The functional significance of ABCC4 transporter in the efflux of MeHg was shown by the ABCC4 inhibition study. Additionally, we identified the role of phospho-eIF2α/ATF4 pathway in the upregulation of LAT1, SNAT2, and ABCC4 and the role of NMD suppression in LAT3 upregulation. Further, we detected that ER stress preconditioning amplified membrane transporter expression most likely through the translation of the upregulated mRNAs caused by ATF4-dependent transcription and NMD suppression. Taken together, these results suggested that the phospho-eIF2α/ATF4 pathway activation and NMD suppression may represent therapeutic targets for the alleviation of MeHg cytotoxicity by enhancing mercury efflux besides inducing protective stress responses.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Environmental stresses suppress nonsense-mediated mRNA decay (NMD) and affect cells by stabilizing NMD-targeted gene expression.Sci Rep. 2019 Feb 4;9(1):1279. doi: 10.1038/s41598-018-38015-2. Sci Rep. 2019. PMID: 30718659 Free PMC article.
-
Endoplasmic reticulum stress preconditioning attenuates methylmercury-induced cellular damage by inducing favorable stress responses.Sci Rep. 2013;3:2346. doi: 10.1038/srep02346. Sci Rep. 2013. PMID: 23907635 Free PMC article.
-
Methylmercury activates ASK1/JNK signaling pathways, leading to apoptosis due to both mitochondria- and endoplasmic reticulum (ER)-generated processes in myogenic cell lines.Neurotoxicology. 2008 Jan;29(1):22-30. doi: 10.1016/j.neuro.2007.08.011. Epub 2007 Sep 1. Neurotoxicology. 2008. PMID: 17920127
-
Methylmercury exposure induces ROS/Akt inactivation-triggered endoplasmic reticulum stress-regulated neuronal cell apoptosis.Toxicology. 2019 Sep 1;425:152245. doi: 10.1016/j.tox.2019.152245. Epub 2019 Jul 19. Toxicology. 2019. PMID: 31330229
-
Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System.Antioxidants (Basel). 2020 Oct 16;9(10):1004. doi: 10.3390/antiox9101004. Antioxidants (Basel). 2020. PMID: 33081221 Free PMC article. Review.
Cited by
-
Long Noncoding RNAs and Circular RNAs Regulate AKT and Its Effectors to Control Cell Functions of Cancer Cells.Cells. 2022 Sep 20;11(19):2940. doi: 10.3390/cells11192940. Cells. 2022. PMID: 36230902 Free PMC article. Review.
-
Involvement of human and canine MRP1 and MRP4 in benzylpenicillin transport.PLoS One. 2019 Nov 27;14(11):e0225702. doi: 10.1371/journal.pone.0225702. eCollection 2019. PLoS One. 2019. PMID: 31774876 Free PMC article.
-
Environmental stresses suppress nonsense-mediated mRNA decay (NMD) and affect cells by stabilizing NMD-targeted gene expression.Sci Rep. 2019 Feb 4;9(1):1279. doi: 10.1038/s41598-018-38015-2. Sci Rep. 2019. PMID: 30718659 Free PMC article.
-
Specific MRP4 Inhibitor Ceefourin-1 Enhances Apoptosis Induced by 6-Mercaptopurine in Jurkat Leukemic Cells, but Not in Normal Lymphoblast Cell Line CRL-1991.Medicina (Kaunas). 2022 May 24;58(6):695. doi: 10.3390/medicina58060695. Medicina (Kaunas). 2022. PMID: 35743958 Free PMC article.
-
Alternative Splicing in Tumorigenesis and Cancer Therapy.Biomolecules. 2025 May 29;15(6):789. doi: 10.3390/biom15060789. Biomolecules. 2025. PMID: 40563429 Free PMC article. Review.
References
-
- Park ST, Lim KT, Chung YT, Kim SU. Methylmercury-induced neurotoxicity in cerebral neuron culture is blocked by antioxidants and NMDA receptor antagonists. Neurotoxicology. 1996;17:37–45. - PubMed
-
- Yee S, Choi BH. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 1996;17:17–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

