Naturally occurring capsid protein variants L1 of human papillomavirus genotype 16 in Morocco
- PMID: 28959092
- PMCID: PMC5609288
- DOI: 10.6026/97320630013241
Naturally occurring capsid protein variants L1 of human papillomavirus genotype 16 in Morocco
Abstract
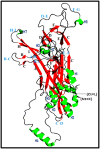
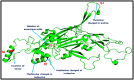
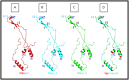
HPV L1 protein is a corner stone in HPV structure, it's involved in the formation of the viral capsid; widely used as a systematic material and considered as the main component in vaccines development and production. The present study aims to characterize genetic variation of L1 gene of HPV 16 specimens and to evaluate in silico the impact of major variants on the epitope change affecting its conformational structure. A fragment of L1 gene from 35 HPV 16 confirmed specimens were amplified by PCR and sequenced. Overall, five amino acids residues changes were reported: T390P in 16 specimens, M425I and M431I in 2 cases, insertion of Serine at 460 and aspartic acid deletion at position 477 in all analyzed cases. The 3D generated model showed that T389P amino acid substitution is located in the H-I loop; the two substitutions M424I and M430I are both located in the H2 helice. The Serine insertion and aspartic acid deletion are located in the H4 helice and B-C loop, respectively. Superimposition of sequences' structures showed that they share a very similar conformation highlighting that the reported amino acids variations don't affect the structure of the L1 protein. However T389P, located in the H-I loop identified as an immunogenetic region of L1 capsid, was reported in 51.4% of cases could interact with vaccines induced monoclonal antibodies suggesting a potential impact on the efficacy of available anti-HPV vaccines.
Keywords: HPV 16; L1 protein; genetic variation; in silico prediction; vaccine.
Figures

Similar articles
-
Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas.Viruses. 2022 Dec 31;15(1):141. doi: 10.3390/v15010141. Viruses. 2022. PMID: 36680181 Free PMC article. Review.
-
Phylogeny and In Silico Structure Analysis of Major Capsid Protein (L1) Human Papillomavirus 45 from Indonesian Isolates.Asian Pac J Cancer Prev. 2020 Sep 1;21(9):2517-2523. doi: 10.31557/APJCP.2020.21.9.2517. Asian Pac J Cancer Prev. 2020. PMID: 32986347 Free PMC article.
-
Comprehensive Assessment of the Antigenic Impact of Human Papillomavirus Lineage Variation on Recognition by Neutralizing Monoclonal Antibodies Raised against Lineage A Major Capsid Proteins of Vaccine-Related Genotypes.J Virol. 2020 Nov 23;94(24):e01236-20. doi: 10.1128/JVI.01236-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32967963 Free PMC article.
-
Worldwide genetic variations in high-risk human papillomaviruses capsid L1 gene and their impact on vaccine efficiency.Gene. 2021 May 25;782:145533. doi: 10.1016/j.gene.2021.145533. Epub 2021 Feb 23. Gene. 2021. PMID: 33636291 Review.
-
Amino acid sequence diversity of the major human papillomavirus capsid protein: implications for current and next generation vaccines.Infect Genet Evol. 2013 Aug;18:151-9. doi: 10.1016/j.meegid.2013.05.013. Epub 2013 May 27. Infect Genet Evol. 2013. PMID: 23722024 Free PMC article.
Cited by
-
Detecting Human Papillomavirus Type 16 in Cervical Cancer Patients with Molecular Variation of Gene L1 in Riau Province Indonesia.Asian Pac J Cancer Prev. 2022 Jan 1;23(1):87-92. doi: 10.31557/APJCP.2022.23.1.87. Asian Pac J Cancer Prev. 2022. PMID: 35092375 Free PMC article.
-
Abundance of HPV L1 Intra-Genotype Variants With Capsid Epitopic Modifications Found Within Low- and High-Grade Pap Smears With Potential Implications for Vaccinology.Front Genet. 2019 May 24;10:489. doi: 10.3389/fgene.2019.00489. eCollection 2019. Front Genet. 2019. PMID: 31231420 Free PMC article.
-
Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas.Viruses. 2022 Dec 31;15(1):141. doi: 10.3390/v15010141. Viruses. 2022. PMID: 36680181 Free PMC article. Review.
-
Genetic diversity and phylogenetic analysis of HPV 16 & 18 variants isolated from cervical specimens of women in Saudi Arabia.Saudi J Biol Sci. 2019 Feb;26(2):317-324. doi: 10.1016/j.sjbs.2018.05.005. Epub 2018 May 3. Saudi J Biol Sci. 2019. PMID: 31485171 Free PMC article.
-
Molecular Analysis and Bioinformatics Assessment of Full-Length L1 Gene of Bovine Papillomavirus Type-1 as a Potential DNA Vaccine Study.Vet Med Int. 2025 Apr 10;2025:6785087. doi: 10.1155/vmi/6785087. eCollection 2025. Vet Med Int. 2025. PMID: 40255612 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
