The Role of Endoglin in Myocardial Fibrosis
- PMID: 28959097
- PMCID: PMC5611341
- DOI: 10.6515/acs20170221b
The Role of Endoglin in Myocardial Fibrosis
Abstract
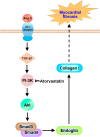
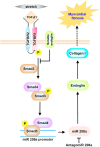
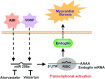
Myocardial fibrosis is closely associated with heart failure because myocardial fibrosis may cause the loss of normal cardiac function. Endoglin is a homeodimeric membrane glycoprotein, a co-receptor of transforming growth factor-β1 (TGF-β1) and β3. Endoglin is a potent mediator of profibrotic effects of angiotensin II on cardiac fibroblasts and can modulate the effect of TGF-β1 on extracellular matrix synthesis. These data indicate that endoglin plays an important role in fibrogenesis in cardiac remodeling. Endoglin induced by TGF-β1 is largely through PI-3 kinase, Akt, Smad3/4 and endoglin promoter pathways. Endoglin was upregulated in pressure- overload, volume-overload heart failure and acute myocardial infarction and was associated with myocardial fibrosis. Silencing endoglin expression could attenuate myocardial fibrosis and improve survival in animal study. Endoglin expression was increased in failing left ventricle before use of left ventricle assist device, and reduced back to control levels after use of left ventricle assist device. Targeting endoglin may provide a potentially unique and novel therapeutic approach for reducing myocardial fibrosis in patients with heart failure.
Keywords: Angiotensin II; Endoglin; MicroRNA; Myocardial fibrosis; TGF-β1.
Figures
References
-
- Aronow WS. Epidemiology, pathophysiology, prognosis, and treatment of systolic and diastolic heart failure. Cardiol Rev. 2006;14:108–124. - PubMed
-
- Mozaflarian D, Benzamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update. A report from the American Heart Association. Circulation. 2016;133:e38–e360. - PubMed
-
- Sutton MGSJ, Sharpe N. Left ventricular remodeling after myocardial infarction. Pathophysiology and therapy. Circulation. 2000;101:2981–2988. - PubMed
-
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Phys Rev. 1999;79:215–262. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
