Effects of a pre-workout supplement on hyperemia following leg extension resistance exercise to failure with different resistance loads
- PMID: 28959158
- PMCID: PMC5615454
- DOI: 10.1186/s12970-017-0195-6
Effects of a pre-workout supplement on hyperemia following leg extension resistance exercise to failure with different resistance loads
Abstract
Background: We sought to determine if a pre-workout supplement (PWS), containing multiple ingredients thought to enhance blood flow, increases hyperemia associated with resistance training compared to placebo (PBO). Given the potential interaction with training loads/time-under-tension, we evaluated the hyperemic response at two different loads to failure.
Methods: Thirty males participated in this double-blinded study. At visit 1, participants were randomly assigned to consume PWS (Reckless™) or PBO (maltodextrin and glycine) and performed four sets of leg extensions to failure at 30% or 80% of their 1-RM 45-min thereafter. 1-wk. later (visit 2), participants consumed the same supplement as before, but exercised at the alternate load. Heart rate (HR), blood pressure (BP), femoral artery blood flow, and plasma nitrate/nitrite (NOx) were assessed at baseline (BL), 45-min post-PWS/PBO consumption (PRE), and 5-min following the last set of leg extensions (POST). Vastus lateralis near infrared spectroscopy (NIRS) was employed during leg extension exercise. Repeated measures ANOVAs were performed with time, supplement, and load as independent variables and Bonferroni correction applied for multiple post-hoc comparisons. Data are reported as mean ± SD.
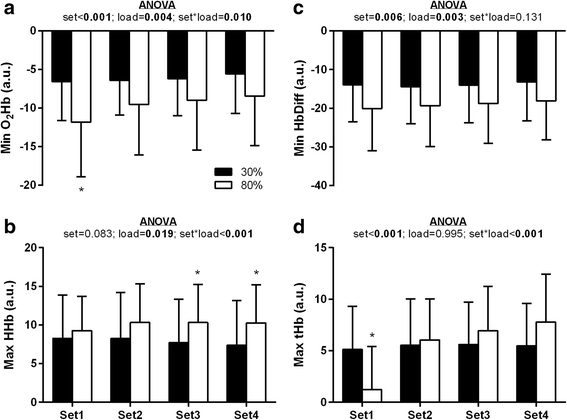
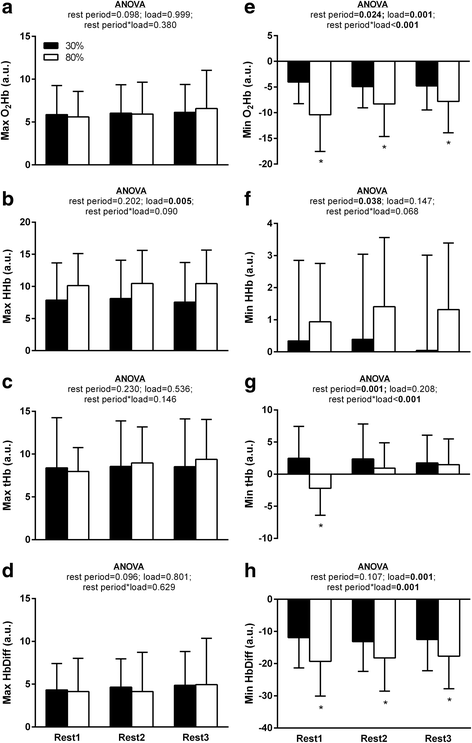
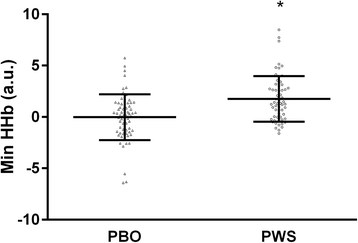
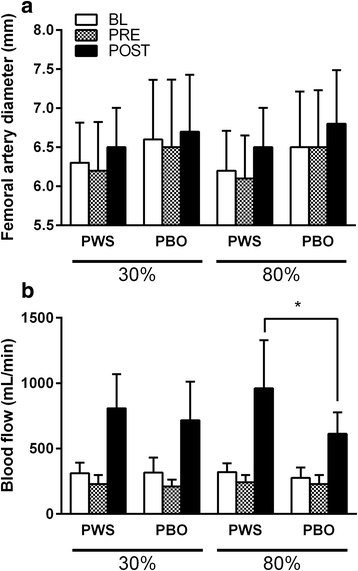
Results: With the 30% training load compared to 80%, significantly more repetitions were performed (p < 0.05), but there was no difference in total volume load (p > 0.05). NIRS derived minimum oxygenated hemoglobin (O2Hb) was lower in the 80% load condition compared to 30% for all rest intervals between sets of exercise (p < 0.0167). HR and BP did not vary as a function of supplement or load. Femoral artery blood flow at POST was higher independent of exercise load and treatment. However, a time*supplement*load interaction was observed revealing greater femoral artery blood flow with PWS compared to PBO at POST in the 80% (+56.8%; p = 0.006) but not 30% load condition (+12.7%; p = 0.476). Plasma NOx was ~3-fold higher with PWS compared to PBO at PRE and POST (p < 0.001).
Conclusions: Compared to PBO, the PWS consumed herein augmented hyperemia following multiple sets to failure at 80% of 1-RM, but not 30%. This specificity may be a product of interaction with local perturbations (e.g., reduced tissue oxygenation levels [minimum O2Hb] in the 80% load condition) and/or muscle fiber recruitment.
Keywords: Blood flow; Nitric oxide, Volume load; Oxygenation; Reactive hyperemia; Resistance exercise; Sports supplements.
Conflict of interest statement
Ethics approval and consent to participate
All procedures described herein were approved by the Auburn University Institutional Review Board (Protocol #16–333 MR 1609) and conformed to the standards set by the latest revision of the Declaration of Helsinki. All subjects provided written and verbal consent prior to study participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
