Electrical Signaling, Photosynthesis and Systemic Acquired Acclimation
- PMID: 28959209
- PMCID: PMC5603676
- DOI: 10.3389/fphys.2017.00684
Electrical Signaling, Photosynthesis and Systemic Acquired Acclimation
Abstract
Electrical signaling in higher plants is required for the appropriate intracellular and intercellular communication, stress responses, growth and development. In this review, we have focus on recent findings regarding the electrical signaling, as a major regulator of the systemic acquired acclimation (SAA) and the systemic acquired resistance (SAR). The electric signaling on its own cannot confer the required specificity of information to trigger SAA and SAR, therefore, we have also discussed a number of other mechanisms and signaling systems that can operate in combination with electric signaling. We have emphasized the interrelation between ionic mechanism of electrical activity and regulation of photosynthesis, which is intrinsic to a proper induction of SAA and SAR. In a special way, we have summarized the role of non-photochemical quenching and its regulator PsbS. Further, redox status of the cell, calcium and hydraulic waves, hormonal circuits and stomatal aperture regulation have been considered as components of the signaling. Finally, a model of light-dependent mechanisms of electrical signaling propagation has been presented together with the systemic regulation of light-responsive genes encoding both, ion channels and proteins involved in regulation of their activity. Due to space limitations, we have not addressed many other important aspects of hormonal and ROS signaling, which were presented in a number of recent excellent reviews.
Keywords: PsbS overexpression and npq-4; electrical signal; ion channel activity; photosynthesis; plasma membrane.
Figures

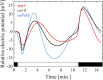
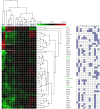
References
-
- Agosti R. D. (2014). Touch-induced action potentials in Arabidopsis thaliana. Arch. Sci. 67, 125–138. Available online at: https://archive-ouverte.unige.ch/unige:48136
-
- Baluška Ed. (2013). Long-Distance Systemic Signaling and Communication in Plants Berlin; Heidelberg: Springer-Verlag.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

