Both 3,5-Diiodo-L-Thyronine and 3,5,3'-Triiodo-L-Thyronine Prevent Short-term Hepatic Lipid Accumulation via Distinct Mechanisms in Rats Being Fed a High-Fat Diet
- PMID: 28959215
- PMCID: PMC5603695
- DOI: 10.3389/fphys.2017.00706
Both 3,5-Diiodo-L-Thyronine and 3,5,3'-Triiodo-L-Thyronine Prevent Short-term Hepatic Lipid Accumulation via Distinct Mechanisms in Rats Being Fed a High-Fat Diet
Abstract
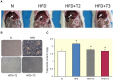
3,3',5-triiodo-L-thyronine (T3) improves hepatic lipid accumulation by increasing lipid catabolism but it also increases lipogenesis, which at first glance appears contradictory. Recent studies have shown that 3,5-diiodothyronine (T2), a natural thyroid hormone derivative, also has the capacity to stimulate hepatic lipid catabolism, however, little is known about its possible effects on lipogenic gene expression. Because genes classically involved in hepatic lipogenesis such as SPOT14, acetyl-CoA-carboxylase (ACC), and fatty acid synthase (FAS) contain thyroid hormone response elements (TREs), we studied their transcriptional regulation, focusing on TRE-mediated effects of T3 compared to T2 in rats receiving high-fat diet (HFD) for 1 week. HFD rats showed a marked lipid accumulation in the liver, which was significantly reduced upon simultaneous administration of either T3 or T2 with the diet. When administered to HFD rats, T2, in contrast with T3, markedly downregulated the expression of the above-mentioned genes. T2 downregulated expression of the transcription factors carbohydrate-response element-binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c) involved in activation of transcription of these genes, which explains the suppressed expression of their target genes involved in lipogenesis. T3, however, did not repress expression of the TRE-containing ChREBP gene but repressed SREBP-1c expression. Despite suppression of SREBP-1c expression by T3 (which can be explained by the presence of nTRE in its promoter), the target genes were not suppressed, but normalized to HFD reference levels or even upregulated (ACC), partly due to the presence of TREs on the promoters of these genes and partly to the lack of suppression of ChREBP. Thus, T2 and T3 probably act by different molecular mechanisms to achieve inhibition of hepatic lipid accumulation.
Keywords: lipid metabolism; lipogenesis; thyroid hormone response element; thyroid hormones; thyroid hormones receptors.
Figures
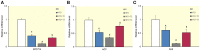


Similar articles
-
3,5-diiodo-L-thyronine increases de novo lipogenesis in liver from hypothyroid rats by SREBP-1 and ChREBP-mediated transcriptional mechanisms.IUBMB Life. 2019 Jul;71(7):863-872. doi: 10.1002/iub.2014. Epub 2019 Feb 1. IUBMB Life. 2019. PMID: 30707786
-
3,5-Diiodo-l-thyronine induces SREBP-1 proteolytic cleavage block and apoptosis in human hepatoma (Hepg2) cells.Biochim Biophys Acta. 2013 Dec;1831(12):1679-89. doi: 10.1016/j.bbalip.2013.08.003. Epub 2013 Aug 13. Biochim Biophys Acta. 2013. PMID: 23948263
-
Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats.Diabetes. 2011 Nov;60(11):2730-9. doi: 10.2337/db11-0207. Epub 2011 Sep 16. Diabetes. 2011. PMID: 21926273 Free PMC article.
-
Action of Thyroid Hormones, T3 and T2, on Hepatic Fatty Acids: Differences in Metabolic Effects and Molecular Mechanisms.Int J Mol Sci. 2017 Mar 31;18(4):744. doi: 10.3390/ijms18040744. Int J Mol Sci. 2017. PMID: 28362337 Free PMC article. Review.
-
Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c.Diabetes Obes Metab. 2010 Oct;12 Suppl 2:83-92. doi: 10.1111/j.1463-1326.2010.01275.x. Diabetes Obes Metab. 2010. PMID: 21029304 Review.
Cited by
-
miR-22-3p is involved in gluconeogenic pathway modulated by 3,5-diiodo-L-thyronine (T2).Sci Rep. 2019 Nov 12;9(1):16645. doi: 10.1038/s41598-019-53019-2. Sci Rep. 2019. PMID: 31719576 Free PMC article.
-
Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals.Int J Mol Sci. 2020 Jun 10;21(11):4140. doi: 10.3390/ijms21114140. Int J Mol Sci. 2020. PMID: 32532017 Free PMC article. Review.
-
Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options.Int J Mol Sci. 2020 Aug 18;21(16):5927. doi: 10.3390/ijms21165927. Int J Mol Sci. 2020. PMID: 32824723 Free PMC article. Review.
-
Hypothyroidism and Nonalcoholic Fatty Liver Disease: Pathophysiological Associations and Therapeutic Implications.J Clin Transl Hepatol. 2020 Sep 28;8(3):347-353. doi: 10.14218/JCTH.2020.00027. Epub 2020 Jul 21. J Clin Transl Hepatol. 2020. PMID: 33083258 Free PMC article. Review.
-
Novel insights into the pathological development of dyslipidemia in patients with hypothyroidism.Bosn J Basic Med Sci. 2022 Jun 1;22(3):326-339. doi: 10.17305/bjbms.2021.6606. Bosn J Basic Med Sci. 2022. PMID: 34784265 Free PMC article. Review.
References
-
- Antonelli A., Fallahi P., Ferrari S. M., Di Domenicantonio A., Moreno M., Lanni A., et al. . (2011). 3,5-diiodo-L-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J. Biol. Regul. Homeost. Agents 25, 655–660. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

