Target-triggered cascade assembly of a catalytic network as an artificial enzyme for highly efficient sensing
- PMID: 28959405
- PMCID: PMC5602372
- DOI: 10.1039/c7sc01453h
Target-triggered cascade assembly of a catalytic network as an artificial enzyme for highly efficient sensing
Abstract
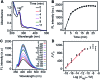
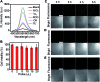
Determining the catalytic activity of artificial enzymes is an ongoing challenge. In this work, we design a porphyrin-based enzymatic network through the target-triggered cascade assembly of catalytic nanoparticles. The nanoparticles are synthesized via the covalent binding of hemin to amino-coated gold nanoparticles and then the axial coordination of the Fe center with a dual-functional imidazole or pyridine derivative. The network, which is specifically formed by coordination polymerization triggered by Hg2+ as the target, shows high catalytic activity due to the triple amplification of enzymatic activity during the cascade assembly. The catalytic dynamics are comparable to those of natural horseradish peroxidase. The catalytic characteristics can be ultrasensitively regulated by the target, leading to a selective methodology for the analysis of sub-attomolar Hg2+. It has also been used for "signal-on" imaging of reactive oxygen species in living cells. This work provides a new avenue for the design of enzyme mimics, and a powerful biocatalyst with signal switching for the development of biosensing protocols.
Figures






Similar articles
-
Highly sensitive magnetic relaxation sensing method for aflatoxin B1 detection based on Au NP-assisted triple self-assembly cascade signal amplification.Biosens Bioelectron. 2021 Nov 15;192:113489. doi: 10.1016/j.bios.2021.113489. Epub 2021 Jul 9. Biosens Bioelectron. 2021. PMID: 34293688
-
Enzyme-triggered formation of enzyme-tyramine concatamers on nanogold-functionalized dendrimer for impedimetric detection of Hg(II) with sensitivity enhancement.Biosens Bioelectron. 2016 Jan 15;75:108-15. doi: 10.1016/j.bios.2015.08.026. Epub 2015 Aug 15. Biosens Bioelectron. 2016. PMID: 26301998
-
A convenient and economical strategy for multiple-target electrochemiluminescence detection using peroxydisulfate solution.Talanta. 2023 Jan 1;251:123788. doi: 10.1016/j.talanta.2022.123788. Epub 2022 Aug 1. Talanta. 2023. PMID: 35933847
-
Enzyme Mimic Based on a Self-Assembled Chitosan/DNA Hybrid Exhibits Superior Activity and Tolerance.Chemistry. 2019 Sep 25;25(54):12576-12582. doi: 10.1002/chem.201902509. Epub 2019 Sep 3. Chemistry. 2019. PMID: 31314132
-
Hemin/G-Quadruplex Horseradish Peroxidase-Mimicking DNAzyme: Principle and Biosensing Application.Adv Biochem Eng Biotechnol. 2020;170:85-106. doi: 10.1007/10_2017_37. Adv Biochem Eng Biotechnol. 2020. PMID: 29143069 Review.
References
-
- Beauchemin A. M. Nat. Chem. 2013;5:731–732. - PubMed
-
- Fischlechner M., Schaerli Y., Mohamed M. F., Patil S., Abell C., Hollfelder F. Nat. Chem. 2014;6:791–796. - PubMed
-
- Ogi S., Sugiyasu K., Manna S., Samitsu S., Takeuchi M. Nat. Chem. 2014;6:188–195. - PubMed
-
- Bruice T. C. Acc. Chem. Res. 1991;24:243–249.
LinkOut - more resources
Full Text Sources
Other Literature Sources

