Imaging Features of Toxicities by Immune Checkpoint Inhibitors in Cancer Therapy
- PMID: 28959504
- PMCID: PMC5594046
- DOI: 10.1007/s40134-017-0256-2
Imaging Features of Toxicities by Immune Checkpoint Inhibitors in Cancer Therapy
Abstract
Purpose of review: With the increasing use of immune checkpoint inhibitors in cancer therapy radiographic profiling of frequent and serious immune-related adverse events (irAEs) becomes more relevant. This article reviews imaging features of irAEs induced by the anti-CTLA-4 and anti-PD-1 antibodies ipilimumab, nivolumab and pembrolizumab.
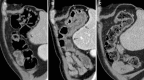
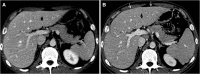
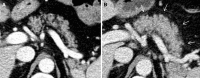
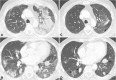
Recent findings: Important radiological manifestations are immune-related colitis, hepatitis, pancreatitis, hypophysitis, pneumonitis, arthritis and sarcoid-like lymphadenopathy. Typical imaging features are summarized and compared with other relevant differential diagnoses.
Summary: Early diagnosis and appropriate therapeutic decisions are required for a successful treatment of irAEs. In addition to staging and follow-up imaging, identification and monitoring of adverse events becomes an important radiologic aspect in oncologic care.
Keywords: Imaging; Immune checkpoint inhibitors; Immune-related adverse events.
Conflict of interest statement
Conflict of Interest
Gerlig Widmann, Van Anh Nguyen, Julian Plaickner and Werner Jaschke each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures
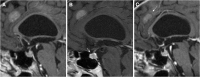
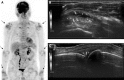
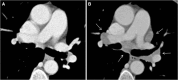
Similar articles
-
[Adverse effects of immunotherapy : Clinical aspects, radiological and nuclear medicine results].Radiologe. 2017 Oct;57(10):840-849. doi: 10.1007/s00117-017-0285-0. Radiologe. 2017. PMID: 28733704 Review. German.
-
Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab.Cancer Immunol Res. 2015 Oct;3(10):1185-92. doi: 10.1158/2326-6066.CIR-15-0102. Epub 2015 Jun 22. Cancer Immunol Res. 2015. PMID: 26100356 Free PMC article.
-
Immune checkpoint blocker-related sarcoid-like granulomatous inflammation: a rare adverse event detected in lymph node aspiration cytology of patients treated for advanced malignant melanoma.Hum Pathol. 2019 Sep;91:69-76. doi: 10.1016/j.humpath.2019.07.001. Epub 2019 Jul 4. Hum Pathol. 2019. PMID: 31279873
-
Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents.Oncologist. 2016 Oct;21(10):1230-1240. doi: 10.1634/theoncologist.2016-0055. Epub 2016 Jul 8. Oncologist. 2016. PMID: 27401894 Free PMC article. Review.
-
Immune-related adverse events on body CT in patients with small-cell lung cancer treated with immune-checkpoint inhibitors.Eur J Radiol. 2020 Nov;132:109275. doi: 10.1016/j.ejrad.2020.109275. Epub 2020 Sep 10. Eur J Radiol. 2020. PMID: 32949913 Free PMC article.
Cited by
-
Immunotherapy-related adverse effects on 18F-FDG PET/CT imaging.Br J Radiol. 2020 Jul;93(1111):20190832. doi: 10.1259/bjr.20190832. Epub 2020 Feb 27. Br J Radiol. 2020. PMID: 32105505 Free PMC article.
-
Differential Diagnosis and Clinical Management of a Case of COVID-19 in a Patient With Stage III Lung Cancer Treated With Radio-chemotherapy and Durvalumab.Clin Lung Cancer. 2020 Nov;21(6):e547-e550. doi: 10.1016/j.cllc.2020.05.027. Epub 2020 Jun 2. Clin Lung Cancer. 2020. PMID: 32527714 Free PMC article. No abstract available.
-
Focus on Immune Checkpoint Inhibitors-related Intestinal Inflammation: From Pathogenesis to Therapeutical Approach.Inflamm Bowel Dis. 2024 Jun 3;30(6):1018-1031. doi: 10.1093/ibd/izad229. Inflamm Bowel Dis. 2024. PMID: 37801695 Free PMC article. Review.
-
Immunotherapy-induced Hepatotoxicity: A Review.J Clin Transl Hepatol. 2022 Dec 28;10(6):1194-1204. doi: 10.14218/JCTH.2022.00105. Epub 2022 Jul 22. J Clin Transl Hepatol. 2022. PMID: 36381098 Free PMC article. Review.
-
Predictive Biomarkers for Immune-Related Endocrinopathies following Immune Checkpoint Inhibitors Treatment.Cancers (Basel). 2023 Jan 6;15(2):375. doi: 10.3390/cancers15020375. Cancers (Basel). 2023. PMID: 36672324 Free PMC article. Review.
References
-
- Dyck L, Mills KH. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017 Apr 09. - PubMed
-
- •• Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. (Review of immune-related adverse events rates and managment of toxicities.) - PubMed
-
- Demlova R, Valik D, Obermannova R, ZdraZilova-Dubska L. The safety of therapeutic monoclonal antibodies: implications for cancer therapy including immuno-checkpoint inhibitors. Physiol Res. 2016;65(Supplementum 4):S455–S62. - PubMed
-
- • Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. Ajr. 2011;197(6):W992–W1000. )Case review of 20 patients with radiologic manifestations of immune-related adverse events.) - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
