Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia
- PMID: 28959554
- PMCID: PMC5615837
- DOI: 10.1016/j.toxrep.2016.03.001
Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia
Abstract
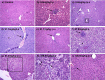
EGCG (Epigallocatechin-3-gallate) is the major active principle catechin found in green tea. Skepticism regarding the safety of consuming EGCG is gaining attention, despite the fact that it is widely being touted for its potential health benefits, including anti-cancer properties. The lack of scientific data on safe dose levels of pure EGCG is of concern, while EGCG has been commonly studied as a component of GTE (Green tea extract) and not as a single active constituent. This study has been carried out to estimate the maximum tolerated non-toxic dose of pure EGCG and to identify the treatment related risk factors. In a fourteen day consecutive treatment, two different administration modalities were compared, offering an improved [i.p (intraperitoneal)] and limited [p.o (oral)] bioavailability. A trend of dose and route dependant hepatotoxicity was observed particularly with i.p treatment and EGCG increased serum lipid profile in parallel to hepatotoxicity. Fourteen day tolerable dose of EGCG was established as 21.1 mg/kg for i.p and 67.8 mg/kg for p.o. We also observed that, EGCG induced effects by both treatment routes are reversible, subsequent to an observation period for further fourteen days after cessation of treatment. It was demonstrated that the severity of EGCG induced toxicity appears to be a function of dose, route of administration and period of treatment.
Keywords: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Dose dependant toxicity; Dyslipidemia; EGCG; GTE, green tea extract; Green tea; Liver toxicity; Route dependant toxicity; Serum lipids; i.p, intraperitoneal; i.v, intravenous; p.o, oral.
Figures


References
-
- Chang P.Y., Mirsalis J., Riccio E.S., Bakke J.P., Lee P.S., Shimon J., Phillips S., Fairchild D., Hara Y., Crowell J.A. Genotoxicity and toxicity of the potential cancer-preventive agent polyphenon E. Environ. Mol. Mutagen. 2003;411:43–54. - PubMed
-
- Chen J.H., Tipoe G.L., Liong E.C., So H.S., Leung K.M., Tom W.M., Fung P.C., Nanji A.A. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am. J. Clin. Nutr. 2004;803:742–751. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

