Comparative Analysis of Bacterial Community Composition and Structure in Clinically Symptomatic and Asymptomatic Central Venous Catheters
- PMID: 28959736
- PMCID: PMC5615130
- DOI: 10.1128/mSphere.00146-17
Comparative Analysis of Bacterial Community Composition and Structure in Clinically Symptomatic and Asymptomatic Central Venous Catheters
Abstract
Totally implanted venous access ports (TIVAPs) are commonly used catheters for the management of acute or chronic pathologies. Although these devices improve health care, repeated use of this type of device for venous access over long periods of time is also associated with risk of colonization and infection by pathogenic bacteria, often originating from skin. However, although the skin microbiota is composed of both pathogenic and nonpathogenic bacteria, the extent and the consequences of TIVAP colonization by nonpathogenic bacteria have rarely been studied. Here, we used culture-dependent and 16S rRNA gene-based culture-independent approaches to identify differences in bacterial colonization of TIVAPs obtained from two French hospitals. To explore the relationships between nonpathogenic organisms colonizing TIVAPs and the potential risk of infection, we analyzed the bacterial community parameters between TIVAPs suspected (symptomatic) or not (asymptomatic) of infection. Although we did not find a particular species assemblage or community marker to distinguish infection risk on an individual sample level, we identified differences in bacterial community composition, diversity, and structure between clinically symptomatic and asymptomatic TIVAPs that could be explored further. This study therefore provides a new view of bacterial communities and colonization patterns in intravascular TIVAPs and suggests that microbial ecology approaches could improve our understanding of device-associated infections and could be a prognostic tool to monitor the evolution of bacterial communities in implants and their potential susceptibility to infections. IMPORTANCE Totally implanted venous access ports (TIVAPs) are commonly used implants for the management of acute or chronic pathologies. Although their use improves the patient's health care and quality of life, they are associated with a risk of infection and subsequent clinical complications, often leading to implant removal. While all TIVAPs appear to be colonized, only a fraction become infected, and the relationship between nonpathogenic organisms colonizing TIVAPs and the potential risk of infection is unknown. We explored bacteria present on TIVAPs implanted in patients with or without signs of TIVAP infection and identified differences in phylum composition and community structure. Our data suggest that the microbial ecology of intravascular devices could be predictive of TIVAP infection status and that ultimately a microbial ecological signature could be identified as a tool to predict TIVAP infection susceptibility and improve clinical management.
Keywords: bacterial community; biofilm; catheter colonization; ecology.
Figures
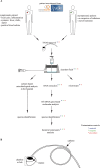


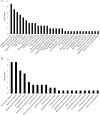
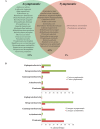
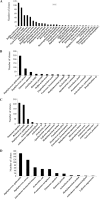
References
-
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
