ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy
- PMID: 28959956
- PMCID: PMC5641217
- DOI: 10.1038/nature24016
ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy
Abstract
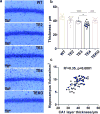
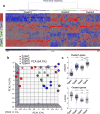
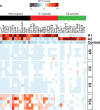
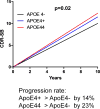
APOE4 is the strongest genetic risk factor for late-onset Alzheimer disease. ApoE4 increases brain amyloid-β pathology relative to other ApoE isoforms. However, whether APOE independently influences tau pathology, the other major proteinopathy of Alzheimer disease and other tauopathies, or tau-mediated neurodegeneration, is not clear. By generating P301S tau transgenic mice on either a human ApoE knock-in (KI) or ApoE knockout (KO) background, here we show that P301S/E4 mice have significantly higher tau levels in the brain and a greater extent of somatodendritic tau redistribution by three months of age compared with P301S/E2, P301S/E3, and P301S/EKO mice. By nine months of age, P301S mice with different ApoE genotypes display distinct phosphorylated tau protein (p-tau) staining patterns. P301S/E4 mice develop markedly more brain atrophy and neuroinflammation than P301S/E2 and P301S/E3 mice, whereas P301S/EKO mice are largely protected from these changes. In vitro, E4-expressing microglia exhibit higher innate immune reactivity after lipopolysaccharide treatment. Co-culturing P301S tau-expressing neurons with E4-expressing mixed glia results in a significantly higher level of tumour-necrosis factor-α (TNF-α) secretion and markedly reduced neuronal viability compared with neuron/E2 and neuron/E3 co-cultures. Neurons co-cultured with EKO glia showed the greatest viability with the lowest level of secreted TNF-α. Treatment of P301S neurons with recombinant ApoE (E2, E3, E4) also leads to some neuronal damage and death compared with the absence of ApoE, with ApoE4 exacerbating the effect. In individuals with a sporadic primary tauopathy, the presence of an ε4 allele is associated with more severe regional neurodegeneration. In individuals who are positive for amyloid-β pathology with symptomatic Alzheimer disease who usually have tau pathology, ε4-carriers demonstrate greater rates of disease progression. Our results demonstrate that ApoE affects tau pathogenesis, neuroinflammation, and tau-mediated neurodegeneration independently of amyloid-β pathology. ApoE4 exerts a 'toxic' gain of function whereas the absence of ApoE is protective.
Figures








Comment in
-
Alzheimer disease: ApoE4 implicated in tau-mediated neurodegeneration.Nat Rev Neurol. 2017 Dec;13(12):706-707. doi: 10.1038/nrneurol.2017.145. Epub 2017 Oct 6. Nat Rev Neurol. 2017. PMID: 28984314 No abstract available.
-
Neurodegeneration: An independent route to toxicity.Nat Rev Neurosci. 2017 Oct 18;18(11):641. doi: 10.1038/nrn.2017.131. Nat Rev Neurosci. 2017. PMID: 29042688 No abstract available.
References
-
- Strittmatter WJ, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG023501/AG/NIA NIH HHS/United States
- R01 NS090934/NS/NINDS NIH HHS/United States
- RF1 NS090934/NS/NINDS NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- RF1 AG047644/AG/NIA NIH HHS/United States
- K08 AG052648/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- R01 AG051812/AG/NIA NIH HHS/United States
- R01 NS088137/NS/NINDS NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- K23 AG055688/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R01 AG054672/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- K01 NS096719/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

