Nanofiber-based sutures induce endogenous antimicrobial peptide
- PMID: 28960168
- PMCID: PMC5674207
- DOI: 10.2217/nnm-2017-0161
Nanofiber-based sutures induce endogenous antimicrobial peptide
Abstract
Aim: The aim of this study was to develop nanofiber-based sutures capable of inducing endogenous antimicrobial peptide production.
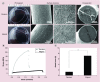
Methods: We used co-axial electrospinning deposition and rolling to fabricate sutures containing pam3CSK4 peptide and 25-hydroxyvitamin D3 (25D3).
Results: The diameters and mechanical properties of the sutures were adjustable to meet the criteria of United States Pharmacopeia designation. 25D3 exhibited a sustained release from nanofiber sutures over 4 weeks. Pam3CSK4 peptide also showed an initial burst followed by a sustained release over 4 weeks. The co-delivery of 25D3 and pam3CSK4 peptide enhanced cathelicidin antimicrobial peptide production from U937 cells and keratinocytes compared with 25D3 delivery alone. In addition, the 25D3/pam3CSK4 peptide co-loaded nanofiber sutures did not significantly influence proliferation of keratinocytes, fibroblasts, or the monocytic cell lines U937 and HL-60.
Conclusion: The use of 25D3/pam3CSK4 peptide co-loaded nanofiber sutures could potentially induce endogenous antimicrobial peptide production and reduce surgical site infections.
Keywords: 25-hydroxyvitamin D3; co-delivery; electrospun nanofibers; pam3Cys-Ser-(Lys)4 peptide; surgical site infection; sutures.
Conflict of interest statement
This work was supported partially from startup funds from University of Nebraska Medical Center, National Institute of General Medical Science (NIGMS) Grant 2P20 GM103480–06 and National Institute of General Medical Science (NIGMS) Grant R01GM123081 of the National Institutes of Health, and Otis Glebe Medical Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures






References
-
- Anderson DJ. Surgical site infections. Infect. Dis. Clin. North Am. 2011;25(1):135–153. - PubMed
-
- National Institute for H and Clinical E. Surgical Site Infection: Prevention And Treatment Of Surgical Site Infection. National Institute for Health and Clinical Excellence; 2008. www.nice.org.uk/guidance/cg74
-
- Astagneau P, Rioux C, Golliot F, Brücker G. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J. Hosp. Infect. 2001;48(4):267–274. - PubMed
-
- Justinger C, Moussavian MR, Schlueter C, et al. Antibiotic coating of abdominal closure sutures and wound infection. Surgery. 2009;145(3):330–334. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
