Regular nicotine intake increased tooth movement velocity, osteoclastogenesis and orthodontically induced dental root resorptions in a rat model
- PMID: 28960194
- PMCID: PMC5709548
- DOI: 10.1038/ijos.2017.34
Regular nicotine intake increased tooth movement velocity, osteoclastogenesis and orthodontically induced dental root resorptions in a rat model
Abstract
Orthodontic forces have been reported to significantly increase nicotine-induced periodontal bone loss. At present, however, it is unknown, which further (side) effects can be expected during orthodontic treatment at a nicotine exposure corresponding to that of an average European smoker. 63 male Fischer344 rats were randomized in three consecutive experiments of 21 animals each (A/B/C) to 3 experimental groups (7 rats, 1/2/3): (A) cone-beam-computed tomography (CBCT); (B) histology/serology; (C) reverse-transcription quantitative real-time polymerase chain reaction (RT-qPCR)/cotinine serology-(1) control; (2) orthodontic tooth movement (OTM) of the first and second upper left molar (NiTi closed coil spring, 0.25 N); (3) OTM with 1.89 mg·kg-1 per day s.c. of L(-)-nicotine. After 14 days of OTM, serum cotinine and IL-6 concentration as well as orthodontically induced inflammatory root resorption (OIIRR), osteoclast activity (histology), orthodontic tooth movement velocity (CBCT, within 14 and 28 days of OTM) and relative gene expression of known inflammatory and osteoclast markers were quantified in the dental-periodontal tissue (RT-qPCR). Animals exposed to nicotine showed significantly heightened serum cotinine and IL-6 levels corresponding to those of regular European smokers. Both the extent of root resorption, osteoclast activity, orthodontic tooth movement and gene expression of inflammatory and osteoclast markers were significantly increased compared to controls with and without OTM under the influence of nicotine. We conclude that apart from increased periodontal bone loss, a progression of dental root resorption and accelerated orthodontic tooth movement are to be anticipated during orthodontic therapy, if nicotine consumption is present. Thus patients should be informed about these risks and the necessity of nicotine abstinence during treatment.
Figures
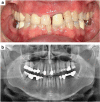


References
-
- Kirschneck C, Meier M, Bauer K et al. Meloxicam medication reduces orthodontically induced dental root resorption and tooth movement velocity: a combined in vivo and in vitro study of dental-periodontal cells and tissue. Cell Tissue Res 2017; 368 (1): 61–78. - PubMed
-
- Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 2006; 28 (3): 221–240. - PubMed
-
- Kanzaki H, Chiba M, Shimizu Y et al. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 2002; 17 (2): 210–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

