Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol
- PMID: 28960802
- PMCID: PMC5871527
- DOI: 10.1111/adb.12551
Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol
Abstract
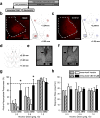
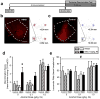
The insular cortex (IC) is a region proposed to modulate, in part, interoceptive states and motivated behavior. Interestingly, IC dysfunction and deficits in interoceptive processing are often found among individuals with substance-use disorders. Furthermore, the IC projects to the nucleus accumbens core (AcbC), a region known to modulate the discriminative stimulus/interoceptive effects of alcohol and other drug-related behaviors. Therefore, the goal of the present work was to investigate the possible role of the IC ➔ AcbC circuit in modulating the interoceptive effects of alcohol. Thus, we utilized a chemogenetic technique (hM4Di designer receptor activation by designer drugs) to silence neuronal activity in the IC of rats trained to discriminate alcohol (1 g/kg, IG) versus water using an operant or Pavlovian alcohol discrimination procedure. Chemogenetic silencing of the IC or IC ➔ AcbC neuronal projections resulted in potentiated sensitivity to the interoceptive effects of alcohol in both the operant and Pavlovian tasks. Together, these data provide critical evidence for the nature of the complex IC circuitry and, specifically, suppression of the insular-striatal circuit in modulating behavior under a drug stimulus control.
Keywords: accumbens; drug discrimination; insula.
© 2017 Society for the Study of Addiction.
Figures

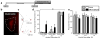
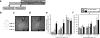
References
-
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. - PubMed
-
- Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. - PubMed
-
- Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW, Jann MW. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:723–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

