Diblock Copolymer Hydrophobicity Facilitates Efficient Gene Silencing and Cytocompatible Nanoparticle-Mediated siRNA Delivery to Musculoskeletal Cell Types
- PMID: 28960967
- PMCID: PMC5736382
- DOI: 10.1021/acs.biomac.7b01349
Diblock Copolymer Hydrophobicity Facilitates Efficient Gene Silencing and Cytocompatible Nanoparticle-Mediated siRNA Delivery to Musculoskeletal Cell Types
Abstract
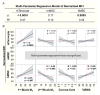
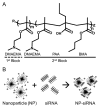
pH-responsive diblock copolymers provide tailorable nanoparticle (NP) architecture and chemistry critical for siRNA delivery. Here, diblock polymers varying in first (corona) and second (core) block molecular weight (Mn), corona/core ratio, and core hydrophobicity (%BMA) were synthesized to determine their effect on siRNA delivery in murine tenocytes (mTenocyte) and murine and human mesenchymal stem cells (mMSC and hMSCs, respectively). NP-mediated siRNA uptake, gene silencing, and cytocompatibility were quantified. Uptake is positively correlated with first block Mn in mTenocytes and hMSCs (p ≤ 0.0005). All NP resulted in significant gene silencing that was positively correlated with %BMA (p < 0.05) in all cell types. Cytocompatibility was reduced in mTenocytes compared to MSCs (p < 0.0001). %BMA was positively correlated with cytocompatibility in MSCs (p < 0.05), suggesting stable NP are more cytocompatible. Overall, this study shows that NP-siRNA cytocompatibility is cell type dependent, and hydrophobicity (%BMA) is the critical diblock copolymer property for efficient gene silencing in musculoskeletal cell types.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Combinatorial optimization of PEG architecture and hydrophobic content improves ternary siRNA polyplex stability, pharmacokinetics, and potency in vivo.J Control Release. 2017 Jun 10;255:12-26. doi: 10.1016/j.jconrel.2017.03.389. Epub 2017 Mar 31. J Control Release. 2017. PMID: 28366646 Free PMC article.
-
Balancing cationic and hydrophobic content of PEGylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo.ACS Nano. 2013 Oct 22;7(10):8870-80. doi: 10.1021/nn403325f. Epub 2013 Sep 23. ACS Nano. 2013. PMID: 24041122 Free PMC article.
-
Development of a novel endosomolytic diblock copolymer for siRNA delivery.J Control Release. 2009 Feb 10;133(3):221-9. doi: 10.1016/j.jconrel.2008.10.004. Epub 2008 Oct 17. J Control Release. 2009. PMID: 18973780 Free PMC article.
-
Synthesis and in vitro characterization of an ABC triblock copolymer for siRNA delivery.Bioconjug Chem. 2007 May-Jun;18(3):736-45. doi: 10.1021/bc060284y. Epub 2007 Mar 15. Bioconjug Chem. 2007. PMID: 17358044
-
Enhancing endosomal escape for nanoparticle mediated siRNA delivery.Nanoscale. 2014 Jun 21;6(12):6415-25. doi: 10.1039/c4nr00018h. Nanoscale. 2014. PMID: 24837409 Review.
Cited by
-
Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery.Nanoscale. 2018 Dec 20;11(1):219-236. doi: 10.1039/c8nr05784b. Nanoscale. 2018. PMID: 30525159 Free PMC article.
-
Degradable poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery.J Control Release. 2018 Oct 10;287:58-66. doi: 10.1016/j.jconrel.2018.08.002. Epub 2018 Aug 3. J Control Release. 2018. PMID: 30077736 Free PMC article.
-
Non-Viral Delivery of Gene Therapy to the Tendon.Polymers (Basel). 2022 Aug 16;14(16):3338. doi: 10.3390/polym14163338. Polymers (Basel). 2022. PMID: 36015594 Free PMC article. Review.
-
Rigor and reproducibility in polymer nanoparticle synthesis and characterization.RSC Adv. 2020;10(5):2513-2518. doi: 10.1039/C9RA10091A. Epub 2020 Jan 14. RSC Adv. 2020. PMID: 34631039 Free PMC article.
-
Recent advances in musculoskeletal local drug delivery.Acta Biomater. 2019 Jul 15;93:135-151. doi: 10.1016/j.actbio.2019.01.043. Epub 2019 Jan 24. Acta Biomater. 2019. PMID: 30685475 Free PMC article. Review.
References
-
- Williford JM, Wu J, Ren Y, Archang MM, Leong KW, Mao HQ. Recent advances in nanoparticle-mediated siRNA delivery. Annu Rev Biomed Eng. 2014;16:347–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

