Spinal Circuits for Touch, Pain, and Itch
- PMID: 28961064
- PMCID: PMC5891508
- DOI: 10.1146/annurev-physiol-022516-034303
Spinal Circuits for Touch, Pain, and Itch
Abstract
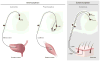
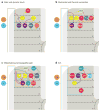
The exteroceptive somatosensory system is important for reflexive and adaptive behaviors and for the dynamic control of movement in response to external stimuli. This review outlines recent efforts using genetic approaches in the mouse to map the spinal cord circuits that transmit and gate the cutaneous somatosensory modalities of touch, pain, and itch. Recent studies have revealed an underlying modular architecture in which nociceptive, pruritic, and innocuous stimuli are processed by distinct molecularly defined interneuron cell types. These include excitatory populations that transmit information about both innocuous and painful touch and inhibitory populations that serve as a gate to prevent innocuous stimuli from activating the nociceptive and pruritic transmission pathways. By dissecting the cellular composition of dorsal-horn networks, studies are beginning to elucidate the intricate computational logic of somatosensory transformation in health and disease.
Keywords: dorsal horn; gate control; interneuron; mechanosensation; nociception; somatosensory system.
Figures
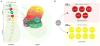
References
-
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 2017;168:295–310. - PMC - PubMed
-
- Alba-Delgado C, El Khoueiry C, Peirs C, Dallel R, Artola A, Antri M. Subpopulations of PKCγ interneurons within the medullary dorsal horn revealed by electrophysiologic and morphologic approach. Pain. 2015;156:1714–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

