Anacetrapib, a New CETP Inhibitor: The New Tool for the Management of Dyslipidemias?
- PMID: 28961179
- PMCID: PMC5750532
- DOI: 10.3390/diseases5040021
Anacetrapib, a New CETP Inhibitor: The New Tool for the Management of Dyslipidemias?
Abstract
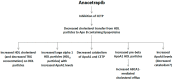
Cholesteryl ester transfer protein (CETP) inhibitors significantly increase serum high-density lipoprotein cholesterol (HDL) cholesterol levels and decrease low-density lipoprotein cholesterol (LDL) cholesterol concentration. However, three drugs of this class failed to show a decrease of cardiovascular events in high-risk patients. A new CETP inhibitor, anacetrapib, substantially increases HDL cholesterol and apolipoprotein (Apo) AI levels with a profound increase of large HDL2 particles, but also pre-β HDL particles, decreases LDL cholesterol levels mainly due to increased catabolism of LDL particles through LDL receptors, decreases lipoprotein a (Lp(a)) levels owing to a decreased Apo (a) production and, finally, decreases modestly triglyceride (TRG) levels due to increased lipolysis and increased receptor-mediated catabolism of TRG-rich particles. Interestingly, anacetrapib may be associated with a beneficial effect on carbohydrate homeostasis. Furthermore, the Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification (REVEAL) trial showed that anacetrapib administration on top of statin treatment significantly reduces cardiovascular events in patients with atherosclerotic vascular disease without any significant increase of adverse events despite its long half-life. Thus, anacetrapib could be useful for the effective management of dyslipidemias in high-risk patients that do not attain their LDL cholesterol target or are statin intolerable, while its role in patients with increased Lp(a) levels remains to be established.
Keywords: anacetrapib; apolipoprotein; cardiovascular disease; cholesteryl ester transfer protein; diabetes.
Conflict of interest statement
This review was conducted independently; no funding was received for writing this manuscript or for covering the costs to publish in open access. M.E. reports personal fees from Astra-Zeneca, grants and personal fees from Merck Sharp & Dohme, personal fees from Pfizer, Abbott, Sanofi-Aventis, Boehringer Ingelheim, Eli-Lilly and GlaxoSmithKline. A.K. and T.F. have given talks and attended conferences sponsored by various pharmaceutical companies, including Bristol-Myers Squibb, Pfizer, Lilly, Abbott, Amgen, AstraZeneca, Novartis, Vianex, Teva and Merck Sharp & Dohme.
Figures


References
-
- Tall A.R. Plasma cholesteryl ester transfer protein. J. Lipid. Res. 1993;34:1255–1274. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

