Transcriptome analysis of hypoxic cancer cells uncovers intron retention in EIF2B5 as a mechanism to inhibit translation
- PMID: 28961236
- PMCID: PMC5636171
- DOI: 10.1371/journal.pbio.2002623
Transcriptome analysis of hypoxic cancer cells uncovers intron retention in EIF2B5 as a mechanism to inhibit translation
Abstract
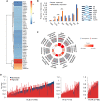
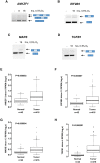
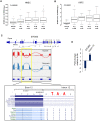
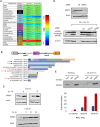
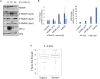
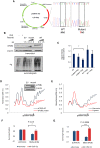
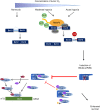
Cells adjust to hypoxic stress within the tumor microenvironment by downregulating energy-consuming processes including translation. To delineate mechanisms of cellular adaptation to hypoxia, we performed RNA-Seq of normoxic and hypoxic head and neck cancer cells. These data revealed a significant down regulation of genes known to regulate RNA processing and splicing. Exon-level analyses classified > 1,000 mRNAs as alternatively spliced under hypoxia and uncovered a unique retained intron (RI) in the master regulator of translation initiation, EIF2B5. Notably, this intron was expressed in solid tumors in a stage-dependent manner. We investigated the biological consequence of this RI and demonstrate that its inclusion creates a premature termination codon (PTC), that leads to a 65kDa truncated protein isoform that opposes full-length eIF2Bε to inhibit global translation. Furthermore, expression of 65kDa eIF2Bε led to increased survival of head and neck cancer cells under hypoxia, providing evidence that this isoform enables cells to adapt to conditions of low oxygen. Additional work to uncover -cis and -trans regulators of EIF2B5 splicing identified several factors that influence intron retention in EIF2B5: a weak splicing potential at the RI, hypoxia-induced expression and binding of the splicing factor SRSF3, and increased binding of total and phospho-Ser2 RNA polymerase II specifically at the intron retained under hypoxia. Altogether, these data reveal differential splicing as a previously uncharacterized mode of translational control under hypoxia and are supported by a model in which hypoxia-induced changes to cotranscriptional processing lead to selective retention of a PTC-containing intron in EIF2B5.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends in molecular medicine. 2012;18(9):534–43. doi: 10.1016/j.molmed.2012.08.001 - DOI - PMC - PubMed
-
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704 - DOI - PubMed
-
- Erapaneedi R, Belousov VV, Schafers M, Kiefer F. A novel family of fluorescent hypoxia sensors reveal strong heterogeneity in tumor hypoxia at the cellular level. Embo j. 2016;35(1):102–13. doi: 10.15252/embj.201592775 - DOI - PMC - PubMed
-
- Brady LK, Popov V, Koumenis C. In Vivo Interrogation of the Hypoxic Transcriptome of Solid Tumors: Optimizing Hypoxic Probe Labeling with Laser Capture Microdissection for Isolation of High-Quality RNA for Deep Sequencing Analysis. Advances in experimental medicine and biology. 2016;899:41–58. doi: 10.1007/978-3-319-26666-4_4 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

