N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes
- PMID: 28961512
- PMCID: PMC5619994
- DOI: 10.1016/j.redox.2017.09.005
N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes
Abstract
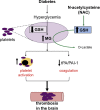
Objective: We previously demonstrated that diabetes exacerbates stroke-induced brain injury, and that this correlates with brain methylglyoxal (MG)-to-glutathione (GSH) status. Cerebral injury was reversed by N-acetylcysteine (NAC). Here we tested if the pro-thrombotic phenotype seen in the systemic circulation and brain during diabetes was associated with increased MG-glycation of proteins, and if NAC could reverse this.
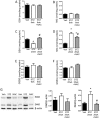
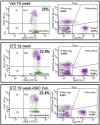
Methods: The streptozotocin (STZ)-induced mouse model of type 1 diabetes was used. Thrombus formation in venules and arterioles (pial circulation) was determined by intravital videomicroscopy using the light-dye method. Circulating blood platelet-leukocyte aggregates (PLAs) were analyzed by flow cytometry 1 wk before other measurements. GSH and MG levels in platelets were measured by HPLC. MG-modified proteins, glutathione peroxidase-1 (GPx-1), and superoxide dismutase-1 (SOD1) levels were detected in platelets by western blot at 20 weeks. Proteins involved in coagulation were quantified by ELISA. NAC (2mM) was given in drinking water for 3 weeks before the terminal experiment.
Results: Thrombus development was accelerated by diabetes in a time-dependent manner. % PLAs were significantly elevated by diabetes. Plasma activated plasminogen activator inhibitor type 1 levels were progressively increased with diabetes duration, with tail bleeding time reduced by 20 wks diabetes. Diabetes lowered platelet GSH levels, GPx-1 and SOD-1 expression. This was associated with higher MG levels, and increased MG-adduct formation in platelets. NAC treatment partly or completely reversed the effects of diabetes.
Conclusion: Collectively, these results show that the diabetic blood and brain become progressively more susceptible to platelet activation and thrombosis. NAC, given after the establishment of diabetes, may offer protection against the risk for stroke by altering both systemic and vascular prothrombotic responses via enhancing platelet GSH, and GSH-dependent MG elimination, as well as correcting levels of antioxidants such as SOD1 and GPx-1.
Keywords: Diabetes; Glutathione; Methylglyoxal; N-acetylcysteine; Thrombosis.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





Similar articles
-
N‑acetylcysteine inhibits atherosclerosis by correcting glutathione‑dependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice.Mol Med Rep. 2021 Mar;23(3):201. doi: 10.3892/mmr.2021.11840. Epub 2021 Jan 26. Mol Med Rep. 2021. PMID: 33495825 Free PMC article.
-
The protection conferred against ischemia-reperfusion injury in the diabetic brain by N-acetylcysteine is associated with decreased dicarbonyl stress.Free Radic Biol Med. 2016 Jul;96:89-98. doi: 10.1016/j.freeradbiomed.2016.03.038. Epub 2016 Apr 12. Free Radic Biol Med. 2016. PMID: 27083477 Free PMC article.
-
Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes.Cardiovasc Diabetol. 2011 May 21;10:43. doi: 10.1186/1475-2840-10-43. Cardiovasc Diabetol. 2011. PMID: 21600014 Free PMC article.
-
Thrombosis in diabetes: a shear flow effect?Clin Sci (Lond). 2017 Jun 15;131(12):1245-1260. doi: 10.1042/CS20160391. Clin Sci (Lond). 2017. PMID: 28592700 Review.
-
Platelet hyperactivity in type 2 diabetes: role of antiplatelet agents.Diab Vasc Dis Res. 2008 Jun;5(2):138-44. doi: 10.3132/dvdr.2008.023. Diab Vasc Dis Res. 2008. PMID: 18537103 Review.
Cited by
-
N-acetylcysteine as a potential treatment for COVID-19.Future Microbiol. 2020 Jul;15:959-962. doi: 10.2217/fmb-2020-0074. Epub 2020 Jul 14. Future Microbiol. 2020. PMID: 32662664 Free PMC article. No abstract available.
-
Preventing the development of severe COVID-19 by modifying immunothrombosis.Life Sci. 2021 Jan 1;264:118617. doi: 10.1016/j.lfs.2020.118617. Epub 2020 Oct 20. Life Sci. 2021. PMID: 33096114 Free PMC article. Review.
-
N-acetylcysteine reduces severity and mortality in COVID-19 patients: A systematic review and meta-analysis.J Adv Vet Anim Res. 2023 Jun 30;10(2):157-168. doi: 10.5455/javar.2023.j665. eCollection 2023 Jun. J Adv Vet Anim Res. 2023. PMID: 37534078 Free PMC article.
-
N-Acetyl-l-Cysteine Supplement in Early Life or Adulthood Reduces Progression of Diabetes in Nonobese Diabetic Mice.Curr Dev Nutr. 2018 Nov 28;3(4):nzy097. doi: 10.1093/cdn/nzy097. eCollection 2019 Apr. Curr Dev Nutr. 2018. PMID: 30993256 Free PMC article.
-
N‑acetylcysteine inhibits atherosclerosis by correcting glutathione‑dependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice.Mol Med Rep. 2021 Mar;23(3):201. doi: 10.3892/mmr.2021.11840. Epub 2021 Jan 26. Mol Med Rep. 2021. PMID: 33495825 Free PMC article.
References
-
- Writing Group M., Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Despres J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jimenez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., Mackey R.H., Magid D.J., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., Turner M.B. American heart association statistics C, stroke statistics S. Executive summary: heart disease and stroke statistics-−2016 update: a report from the american heart association. Circulation. 2016;133:447–454. - PubMed
-
- O'Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., Rangarajan S., Islam S., Pais P., McQueen M.J., Mondo C., Damasceno A., Lopez-Jaramillo P., Hankey G.J., Dans A.L., Yusoff K., Truelsen T., Diener H.C., Sacco R.L., Ryglewicz D., Czlonkowska A., Weimar C., Wang X., Yusuf S., investigators I. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): a case-control study. Lancet. 2010;376:112–123. - PubMed
-
- Singer D.E., Moulton A.W., Nathan D.M. Diabetic myocardial infarction. Interaction of diabetes with other preinfarction risk factors. Diabetes. 1989;38:350–357. - PubMed
-
- McCarron P., Greenwood R., Elwood P., Shlomo Y.B., Bayer A., Baker I., Frankel S., Ebrahim S., Murray L., Smith G.D. The incidence and aetiology of stroke in the caerphilly and speedwell collaborative studies ii: risk factors for ischaemic stroke. Public Health. 2001;115:12–20. - PubMed
-
- Li H.F., Zhang X., Zhang Y., Pan X.D., Zhao H.Q., Li H. Clinical and neuroradiological features of internal watershed infarction and the occlusive diseases of carotid artery system. Neurol. Res. 2010;32:1090–1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

