Insights into the molecular basis of biocontrol of Brassica pathogens by Bacillus amyloliquefaciens UCMB5113 lipopeptides
- PMID: 28961818
- PMCID: PMC5737243
- DOI: 10.1093/aob/mcx089
Insights into the molecular basis of biocontrol of Brassica pathogens by Bacillus amyloliquefaciens UCMB5113 lipopeptides
Abstract
Background and aims: Certain micro-organisms can improve plant protection against pathogens. The protective effect may be direct, e.g. due to antibiotic compounds, or indirect, by priming of plant defence as induced systemic resistance (ISR). The plant growth-promoting rhizobacterium Bacillus amyloliquefaciens UCMB5113 shows potential for disease management of oilseed rape. To investigate the mode of action of this protection, especially in relation to jasmonic acid-dependent ISR, Bacillus UCMB5113 was tested with Arabidopsis thaliana mutants and several important fungal pathogens of Brassica species.
Methods: Secreted lipopeptide fractions from Bacillus UCMB5113, together with synthetic peptide mimics, were evaluated for their effects on fungal phytopathogens and A. thaliana . The structures of secreted lipopeptides were analysed using mass spectrometry. Plant mutants and reporter lines were used to identify signalling steps involved in disease suppression by lipopeptides.
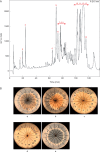
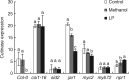
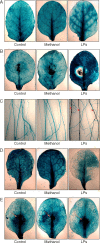
Key results: In plate tests Bacillus UCMB5113 and lipopeptide extracts suppressed growth of several fungal pathogens infecting Brassica plants. Separation of secreted lipopeptides using reversed-phase high-performance liquid chromatography revealed several fractions that inhibited fungal growth. Analysis by mass spectrometry identified the most potent compounds as novel linear forms of antifungal fengycins, with synthetic peptide mimics confirming the biological activity. Application of the lipopeptide extracts on Arabidopsis roots provided systemic protection against Alternaria brassicicola on leaves. Arabidopsis signalling mutants and PDF1.2 and VSP2 promoter-driven GUS lines indicated that the lipopeptide fraction involved jasmonic-acid-dependent host responses for suppression of fungal growth indicative of ISR.
Conclusions: The ability of Bacillus UCMB5113 to counteract pathogens using both antagonistic lipopeptides and through ISR provides a promising tool for sustainable crop production.
Keywords: Antagonism; Arabidopsis thaliana; Bacillus amyloliquefaciens; beneficial bacteria; biocontrol; lipopeptide; rhizosphere.
© The Author 2017. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures



References
-
- Alvarez F, Castro M, Principe A, et al.2012. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of Sclerotinia stem rot disease. Journal of Applied Microbiology 112: 159–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

