Antitumor activity of lurbinectedin (PM01183) and doxorubicin in relapsed small-cell lung cancer: results from a phase I study
- PMID: 28961837
- PMCID: PMC5834091
- DOI: 10.1093/annonc/mdx357
Antitumor activity of lurbinectedin (PM01183) and doxorubicin in relapsed small-cell lung cancer: results from a phase I study
Abstract
Background: Lurbinectedin (PM01183) has synergistic antitumor activity when combined with doxorubicin in mice with xenografted tumors. This phase I trial determined the recommended dose (RD) of doxorubicin (bolus) and PM01183 (1-h intravenous infusion) on day 1 every 3 weeks (q3wk), and obtained preliminary evidence of antitumor activity for this combination in small-cell lung cancer (SCLC).
Patients and methods: Patients with advanced solid tumors received doxorubicin and PM01183 following a standard dose escalation design and expansion at the RD. Twenty-seven patients had relapsed SCLC: 12 with sensitive disease (platinum-free interval ≥90 days) and 15 with resistant disease (platinum-free interval <90 days).
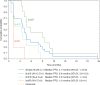
Results: Doxorubicin 50 mg/m2 and PM01183 4.0 mg flat dose was the RD. In relapsed SCLC, treatment tolerance at the RD was manageable. Transient and reversible myelosuppression (including neutropenia, thrombocytopenia, and febrile neutropenia) was the main toxicity, managed with dose adjustment and colony-stimulating factors. Fatigue (79%), nausea/vomiting (58%), decreased appetite (53%), mucositis (53%), alopecia (42%), diarrhea/constipation (42%), and asymptomatic creatinine (68%) and transaminase increases (alanine aminotransferase 42%; aspartate aminotransferase 32%) were common, and mostly mild or moderate. Complete (n = 2, 8%) and partial response (n = 13, 50%) occurred in relapsed SCLC, mostly at the RD. Response rates at second line were 91.7% in sensitive disease [median progression-free survival (PFS)=5.8 months] and 33.3% in resistant disease (median PFS = 3.5 months). At third line, response rate was 20.0% (median PFS = 1.2 months), all in resistant disease.
Conclusion: Doxorubicin 50 mg/m2 and PM01183 4.0 mg flat dose on day 1 q3wk has shown remarkable activity, mainly in second line, with manageable tolerance in relapsed SCLC, leading to further evaluation of this combination within an ongoing phase III trial.
Keywords: PM01183; lurbinectedin; phase I study; small-cell lung cancer.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Similar articles
-
Efficacy and safety of lurbinectedin and doxorubicin in relapsed small cell lung cancer. Results from an expansion cohort of a phase I study.Invest New Drugs. 2021 Oct;39(5):1275-1283. doi: 10.1007/s10637-020-01025-x. Epub 2021 Mar 11. Invest New Drugs. 2021. PMID: 33704620 Free PMC article. Clinical Trial.
-
Phase I clinical and pharmacokinetic study of PM01183 (a tetrahydroisoquinoline, Lurbinectedin) in combination with gemcitabine in patients with advanced solid tumors.Invest New Drugs. 2017 Apr;35(2):198-206. doi: 10.1007/s10637-016-0410-3. Epub 2016 Nov 21. Invest New Drugs. 2017. PMID: 27873130 Clinical Trial.
-
Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial.Lancet Oncol. 2020 May;21(5):645-654. doi: 10.1016/S1470-2045(20)30068-1. Epub 2020 Mar 27. Lancet Oncol. 2020. PMID: 32224306 Clinical Trial.
-
Lurbinectedin is an effective alternative to platinum rechallenge and may restore platinum sensitivity in patients with sensitive relapsed small cell lung cancer.Expert Rev Anticancer Ther. 2025 Jan;25(1):27-40. doi: 10.1080/14737140.2024.2438067. Epub 2024 Dec 12. Expert Rev Anticancer Ther. 2025. PMID: 39660812 Review.
-
Lurbinectedin in the treatment of relapsed small cell lung cancer.Future Oncol. 2021 Jun;17(18):2279-2289. doi: 10.2217/fon-2020-1212. Epub 2021 Mar 19. Future Oncol. 2021. PMID: 33736462 Review.
Cited by
-
Efficacy and safety of lurbinectedin and doxorubicin in relapsed small cell lung cancer. Results from an expansion cohort of a phase I study.Invest New Drugs. 2021 Oct;39(5):1275-1283. doi: 10.1007/s10637-020-01025-x. Epub 2021 Mar 11. Invest New Drugs. 2021. PMID: 33704620 Free PMC article. Clinical Trial.
-
Targeted Therapies in Small Cell Lung Cancer: From Old Failures to Novel Therapeutic Strategies.Int J Mol Sci. 2023 May 17;24(10):8883. doi: 10.3390/ijms24108883. Int J Mol Sci. 2023. PMID: 37240229 Free PMC article. Review.
-
Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin.EBioMedicine. 2021 Jul;69:103456. doi: 10.1016/j.ebiom.2021.103456. Epub 2021 Jul 4. EBioMedicine. 2021. PMID: 34233258 Free PMC article.
-
Real-World Response and Survival Outcomes and Treatment Patterns in Patients With Extensive Stage Small-Cell Lung Cancer Receiving Third-Line Treatment.Cancer Rep (Hoboken). 2025 Aug;8(8):e70289. doi: 10.1002/cnr2.70289. Cancer Rep (Hoboken). 2025. PMID: 40792648 Free PMC article.
-
Self-assembly of DNA nanostructure containing cell-specific aptamer as a precise drug delivery system for cancer therapy in non-small cell lung cancer.J Nanobiotechnology. 2022 Nov 19;20(1):486. doi: 10.1186/s12951-022-01701-5. J Nanobiotechnology. 2022. PMID: 36403038 Free PMC article.
References
-
- Santamaria Nunez G, Robles CM, Giraudon C. et al. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol Cancer Ther 2016; 15: 1–14. - PubMed
-
- Malone H, Atassi G.. DNA topoisomerasa targeting drugs: mechanisms of actions and perspectives. Anticancer Drugs 1997; 8: 811–822. - PubMed
-
- Elez ME, Tabernero J, Geary D. et al. First-in-human phase I study of Lurbinectedin (PM01183) in patients with advanced solid tumors. Clin Cancer Res 2014; 20: 2205–2214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

