Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study
- PMID: 28961848
- PMCID: PMC5834156
- DOI: 10.1093/annonc/mdx339
Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study
Abstract
Background: The BRIM-3 trial showed improved progression-free survival (PFS) and overall survival (OS) for vemurafenib compared with dacarbazine in treatment-naive patients with BRAFV600 mutation-positive metastatic melanoma. We present final OS data from BRIM-3.
Patients and methods: Patients were randomly assigned in a 1 : 1 ratio to receive vemurafenib (960 mg twice daily) or dacarbazine (1000 mg/m2 every 3 weeks). OS and PFS were co-primary end points. OS was assessed in the intention-to-treat population, with and without censoring of data for dacarbazine patients who crossed over to vemurafenib.
Results: Between 4 January 2010 and 16 December 2010, a total of 675 patients were randomized to vemurafenib (n = 337) or dacarbazine (n = 338, of whom 84 crossed over to vemurafenib). At the time of database lock (14 August 2015), median OS, censored at crossover, was significantly longer for vemurafenib than for dacarbazine {13.6 months [95% confidence interval (CI) 12.0-15.4] versus 9.7 months [95% CI 7.9-12.8; hazard ratio (HR) 0.81 [95% CI 0.67-0.98]; P = 0.03}, as was median OS without censoring at crossover [13.6 months (95% CI 12.0-15.4) versus 10.3 months (95% CI 9.1-12.8); HR 0.81 (95% CI 0.68-0.96); P = 0.01]. Kaplan-Meier estimates of OS rates for vemurafenib versus dacarbazine were 56% versus 46%, 30% versus 24%, 21% versus 19% and 17% versus 16% at 1, 2, 3 and 4 years, respectively. Overall, 173 of the 338 patients (51%) in the dacarbazine arm and 175 of the 337 (52%) of those in the vemurafenib arm received subsequent anticancer therapies, most commonly ipilimumab. Safety data were consistent with the primary analysis.
Conclusions: Vemurafenib continues to be associated with improved median OS in the BRIM-3 trial after extended follow-up. OS curves converged after ≈3 years, likely as a result of crossover from dacarbazine to vemurafenib and receipt of subsequent anticancer therapies.
Clinicaltrials.gov: NCT01006980.
Keywords: BRAF mutation; dacarbazine; melanoma; vemurafenib.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
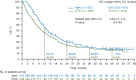

References
-
- Larkin J, Ascierto PA, Dréno B. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371: 1867–1876. - PubMed
-
- Hauschild A, Grob J-J, Demidov LV. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365. - PubMed
-
- Masuda S, Izpisua Belmonte JC.. Trametinib for patients with advanced melanoma. Lancet Oncol 2012; 13: e409–e410. - PubMed
-
- Long GV, Stroyakovskiy D, Gogas H. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371: 1877–1888. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

