Biophysical processes supporting the diversity of microbial life in soil
- PMID: 28961933
- PMCID: PMC5812502
- DOI: 10.1093/femsre/fux039
Biophysical processes supporting the diversity of microbial life in soil
Abstract
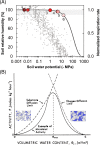
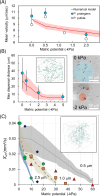
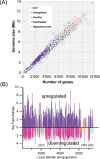
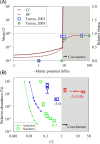
Soil, the living terrestrial skin of the Earth, plays a central role in supporting life and is home to an unimaginable diversity of microorganisms. This review explores key drivers for microbial life in soils under different climates and land-use practices at scales ranging from soil pores to landscapes. We delineate special features of soil as a microbial habitat (focusing on bacteria) and the consequences for microbial communities. This review covers recent modeling advances that link soil physical processes with microbial life (termed biophysical processes). Readers are introduced to concepts governing water organization in soil pores and associated transport properties and microbial dispersion ranges often determined by the spatial organization of a highly dynamic soil aqueous phase. The narrow hydrological windows of wetting and aqueous phase connectedness are crucial for resource distribution and longer range transport of microorganisms. Feedbacks between microbial activity and their immediate environment are responsible for emergence and stabilization of soil structure-the scaffolding for soil ecological functioning. We synthesize insights from historical and contemporary studies to provide an outlook for the challenges and opportunities for developing a quantitative ecological framework to delineate and predict the microbial component of soil functioning.
Keywords: bacterial communities; microbial ecology; microbial interactions; soil microbiology; soil physics; vadose zone.
© FEMS 2017.
Figures



References
-
- Alivisatos AP, Blaser MJ, Brodie EL et al. A unified initiative to harness earth's microbiomes. Science 2015;350:507–8. - PubMed
-
- Allen MF. Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J 2007;6:291–7.
-
- Alster CJ, Koyama A, Johnson NG et al. Temperature sensitivity of soil microbial communities: An application of macromolecular rate theory to microbial respiration. J Geophys Res-Biogeo 2016;121:1420–33.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

