Treatment with AICAR inhibits blastocyst development, trophectoderm differentiation and tight junction formation and function in mice
- PMID: 28962017
- PMCID: PMC5909861
- DOI: 10.1093/molehr/gax050
Treatment with AICAR inhibits blastocyst development, trophectoderm differentiation and tight junction formation and function in mice
Abstract
Study question: What is the impact of adenosine monophosphate-activated protein kinase (AMPK) activation on blastocyst formation, gene expression, and tight junction formation and function?
Summary answer: AMPK activity must be tightly controlled for normal preimplantation development and blastocyst formation to occur.
What is known already: AMPK isoforms are detectable in oocytes, cumulus cells and preimplantation embryos. Cultured embryos are subject to many stresses that can activate AMPK.
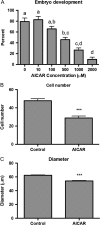
Study design, size, duration: Two primary experiments were carried out to determine the effect of AICAR treatment on embryo development and maintenance of the blastocoel cavity. Embryos were recovered from superovulated mice. First, 2-cell embryos were treated with a concentration series (0-2000 μM) of AICAR for 48 h until blastocyst formation would normally occur. In the second experiment, expanded mouse blastocysts were treated for 9 h with 1000 μM AICAR.
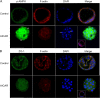
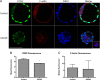
Participants/materials, setting, methods: Outcomes measured included development to the blastocyst stage, cell number, blastocyst volume, AMPK phosphorylation, Cdx2 and blastocyst formation gene family expression (mRNAs and protein measured using quantitative RT-PCR, immunoblotting, immunofluorescence), tight junction function (FITC dextran dye uptake assay), and blastocyst ATP levels. The reversibility of AICAR treatment was assessed using Compound C (CC), a well-known inhibitor of AMPK, alone or in combination with AICAR.
Main results and the role of chance: Prolonged treatment with AICAR from the 2-cell stage onward decreases blastocyst formation, reduces total cell number, embryo diameter, leads to loss of trophectoderm cell contacts and membrane zona occludens-1 staining, and increased nuclear condensation. Treatment with CC alone inhibited blastocyst development only at concentrations that are higher than normally used. AICAR treated embryos displayed altered mRNA and protein levels of blastocyst formation genes. Treatment of blastocysts with AICAR for 9 h induced blastocyst collapse, altered blastocyst formation gene expression, increased tight junction permeability and decreased CDX2. Treated blastocysts displayed three phenotypes: those that were unaffected by treatment, those in which treatment was reversible, and those in which effects were irreversible.
Large scale data: Not applicable.
Limitations, reasons for caution: Our study investigates the effects of AICAR treatment on early development. While AICAR does increase AMPK activity and this is demonstrated in our study, AICAR is not a natural regulator of AMPK activity and some outcomes may result from off target non-AMPK AICAR regulated events. To support our results, blastocyst developmental outcomes were confirmed with two other well-known small molecule activators of AMPK, metformin and phenformin.
Wider implications of the findings: Metformin, an AMPK activator, is widely used to treat type II diabetes and polycystic ovarian disorder (PCOS). Our results indicate that early embryonic AMPK levels must be tightly regulated to ensure normal preimplantation development. Thus, use of metformin should be carefully considered during preimplantation and early post-embryo transfer phases of fertility treatment cycles.
Study funding and competing interest(s): Canadian Institutes of Health Research (CIHR) operating funds. There are no competing interests.
Keywords: AMPK; assisted reproductive technologies; blastocyst formation; embryo culture; preimplantation; stress pathways.
© The Author 2017. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures



Similar articles
-
TEAD4 regulates trophectoderm differentiation upstream of CDX2 in a GATA3-independent manner in the human preimplantation embryo.Hum Reprod. 2022 Jul 30;37(8):1760-1773. doi: 10.1093/humrep/deac138. Hum Reprod. 2022. PMID: 35700449
-
Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts.Hum Reprod. 2017 Jul 1;32(7):1382-1392. doi: 10.1093/humrep/dex087. Hum Reprod. 2017. PMID: 28472298 Free PMC article.
-
AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression.Cell Death Differ. 2017 May;24(5):819-831. doi: 10.1038/cdd.2017.14. Epub 2017 Feb 24. Cell Death Differ. 2017. PMID: 28234358 Free PMC article.
-
Retrospective analysis: reproducibility of interblastomere differences of mRNA expression in 2-cell stage mouse embryos is remarkably poor due to combinatorial mechanisms of blastomere diversification.Mol Hum Reprod. 2018 Jul 1;24(7):388-400. doi: 10.1093/molehr/gay021. Mol Hum Reprod. 2018. PMID: 29746690
-
AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review.Cells. 2021 May 4;10(5):1095. doi: 10.3390/cells10051095. Cells. 2021. PMID: 34064363 Free PMC article.
Cited by
-
Capture and metabolomic analysis of the human endometrial epithelial organoid secretome.Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2026804118. doi: 10.1073/pnas.2026804118. Proc Natl Acad Sci U S A. 2021. PMID: 33876774 Free PMC article.
-
Roles of AMP-Activated Protein Kinase (AMPK) in Mammalian Reproduction.Front Cell Dev Biol. 2020 Nov 19;8:593005. doi: 10.3389/fcell.2020.593005. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33330475 Free PMC article. Review.
-
Metformin in Reproductive Biology.Front Endocrinol (Lausanne). 2018 Nov 22;9:675. doi: 10.3389/fendo.2018.00675. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30524372 Free PMC article. Review.
-
Why AMPK agonists not known to be stressors may surprisingly contribute to miscarriage or hinder IVF/ART.J Assist Reprod Genet. 2018 Aug;35(8):1359-1366. doi: 10.1007/s10815-018-1213-6. Epub 2018 Jun 7. J Assist Reprod Genet. 2018. PMID: 29882092 Free PMC article. Review.
-
Oleic Acid Counters Impaired Blastocyst Development Induced by Palmitic Acid During Mouse Preimplantation Development: Understanding Obesity-Related Declines in Fertility.Reprod Sci. 2020 Nov;27(11):2038-2051. doi: 10.1007/s43032-020-00223-5. Epub 2020 Jun 15. Reprod Sci. 2020. PMID: 32542540 Free PMC article.
References
-
- Barcroft LC, Offenberg H, Thomsen P, Watson AJ. Aquaporin proteins in murine trophectoderm mediate transepithelial water movements during cavitation. Dev Biol 2003;256:342–354. - PubMed
-
- Barcroft LC, Moseley AE, Lingrel JB, Watson AJ. Deletion of the Na/K-ATPase alpha1-subunit gene (Atp1a1) does not prevent cavitation of the preimplantation mouse embryo. Mech Dev 2004;121:417–426. - PubMed
-
- Bedaiwy MA, Miller KF, Goldberg JF, Nelson D, Falcone T. Effect of metformin on mouse embryo development. Fertil Steril 2001;76:1078–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

