Construction and assessment of bio-engineered intervertebral discs
- PMID: 28962105
- PMCID: PMC5609117
- DOI: 10.3892/etm.2017.4764
Construction and assessment of bio-engineered intervertebral discs
Abstract
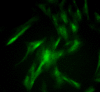
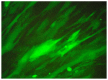
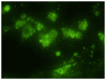
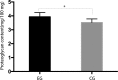
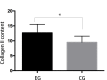
The present study assessed the value of bone marrow-mesenchymal stem cells (BM-MSCs) transformed by nucleus pulposus cells (NPs) for engineering of intervertebral discs. BM-MSCs and fetal NPs were cultured, planted onto polylactic acid-polyglycolic acid co-polymer (PLGA) and observed under inverted and scanning electron microscopes. PLGA scaffolds with adherent or suspended BM-MSCs and NPs were implanted into intervertebral discs of New Zealand white rabbits. Intervertebral signal intensity was evaluated by Thompson grading after 12 weeks. Proteoglycan and type II collagen were measured spectrophotometrically and immunohistochemically, respectively. Spindle or multi-angular BM-MSCs developed fibro-like phenotypesin co-culture with NPs and grew with a normal morphology when attached to PLGA scaffolds. A significant difference was observed in intervertebral proteoglycan expression and collagen II expression in the PLGA scaffold group vs. that in the control group implanted with BM-MSCs and NPs without a scaffold (3.93±0.31 vs. 3.52±0.26 mg/100 mg, 12.70±2.83 vs. 9.50±2.06, respectively). Thus, BM-MSCs can be co-cultured with NPs to enhance their differentiation into NPs for disc regeneration. In conclusion, PLGA scaffolds offer viable growing conditions and allow for the maintenance of mechanical properties and spatial structures of the engineered tissue, which meets the requirements of tissue-engineered discs that do not degenerate.
Keywords: interverbebral disc; mesenchymal stem cells; polylactic acid-polyglycolic acid copolymer; tissue engineering.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
