Establishment of an experimental glaucoma animal model: A comparison of microbead injection with or without hydroxypropyl methylcellulose
- PMID: 28962109
- PMCID: PMC5609141
- DOI: 10.3892/etm.2017.4728
Establishment of an experimental glaucoma animal model: A comparison of microbead injection with or without hydroxypropyl methylcellulose
Abstract
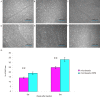
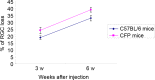
The present study aimed to compare microbead injection with and without hydroxypropyl methylcellulose (HPM) in order to establish an experimental animal model of glaucoma. This model was established in C57BL/6 mice and transgenic mice expressing cyan fluorescent protein (CFP) under the control of the Thy1 promoter in retinal ganglion cells (RGCs). C57BL/6 mice aged between 12 and 20 weeks old were randomly separated into three groups, which received different injections into the anterior chamber of the eye. Group A (microbead) received 2 µl microbeads (10×106 beads/ml) and 1 µl air. Group B (microbeads + HPM) received 2 µl microbeads and 1 µl HPM. Group C (control group) received 2 µl PBS and 1 µl air. The intraocular pressure (IOP) was measured with a tonometer under topical anesthesia daily for 1 month. A single injection of microbeads, with or without HPM, induced consistent IOP elevation when compared with the control group. Thy1-CFP mice received an injection of 2 µl microbeads and 1 µl HPM into the anterior chamber of the eyes, and the number of CFP+ RGCs was subsequently assessed in vivo by confocal scanning laser microscopy in the same area of the retina weekly for 6 weeks. The results from in vivo imaging of Thy1-CFP mice were comparable with the immunohistochemical staining results from the C57BL/6 mice. The combined injection of microbeads and HPM induced longer and higher peaks of IOP elevation when compared with the microbeads alone. The rate of RGC loss following the administration of microbeads alone was 25.0±1.3% 6 weeks after the initial IOP elevation, while it was 33.2±1.9% following the administration of microbeads + HPM. These results indicate that the injection of microbeads + HPM is a more effective method of establishing a mouse model with chronic elevation of IOP. In addition, the in vivo imaging that can be used with this technique provides an effective and noninvasive approach for monitoring the progress of RGC loss.
Keywords: animal model; glaucoma; hydroxypropyl methylcellulose; intraocular pressure; microbead; retinal ganglion cell.
Figures



References
-
- Feng L, Zhao Y, Yoshida M, Chen H, Yang JF, Kim TS, Cang J, Troy JB, Liu X. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Invest Ophthalmol Vis Sci. 2013;54:1106–1117. doi: 10.1167/iovs.12-10791. - DOI - PMC - PubMed
-
- Vidal-Sanz M, Salinas-Navarro M, Nadal-Nicolás FM, Alarcón-Martínez L, Valiente-Soriano FJ, de Imperial JM, Avilés-Trigueros M, Agudo-Barriuso M, Villegas-Pérez MP. Understanding glaucomatous damage: Anatomical and functional data from ocular hypertensive rodent retinas. Prog Retin Eye Res. 2012;31:1–27. doi: 10.1016/j.preteyeres.2011.08.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
