Attenuation of doxorubicin-induced cardiotoxicity by esculetin through modulation of Bmi-1 expression
- PMID: 28962145
- PMCID: PMC5609155
- DOI: 10.3892/etm.2017.4763
Attenuation of doxorubicin-induced cardiotoxicity by esculetin through modulation of Bmi-1 expression
Abstract
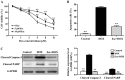
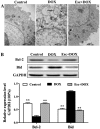
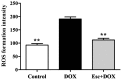
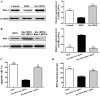
The protective effects and mechanisms of esculetin on doxorubicin (DOX)-induced injury of H9c2 cells were investigated. H9c2 cells were cultured and the logarithmic growth phase of the cells was divided into a control group, a DOX group and an esculetin + DOX group. Cell viability was detected by MTT assay. Annexin V-PI (AV-PI) double staining flow cytometry was carried out to detect cell apoptosis. Intracellular reactive oxygen species (ROS) were detected by flow cytometry. Transmission electron microscope (TEM) was used to evaluate cell ultrastructure. Cleaved caspase-3, cleaved PARP, Bcl-2, Bid and Bmi-1 proteins levels were investigated by western blot analysis. Bmi-1 siRNA was used to detect the role of Bmi-1 in the protective effects of esculetin against DOX-induced toxicity in H9c2 cells. The MTT and AV-PI double staining results showed that esculetin significantly increased H9c2 cell viability. Compared with the control group, the levels of cleaved caspase-3, cleaved PARP, Bid and ROS levels were significantly decreased, but the expression of Bcl-2 and Bmi-1 were significantly increased in the esculetin + DOX group. TEM showed that the cell structure of the mitochondria was protected by esculetin. The results of Bmi-1 siRNA showed that esculetin could protect DOX-induced cardiotoxicity by modulating Bmi-1 expression. Esculetin can protect DOX-induced cardiotoxicity and the effects may be attributable to modulation of Bmi-1 expression, provoking intracellular ROS accumulation, protecting the structure of mitochondria and reducing cell apoptosis.
Keywords: Bmi-1; H9c2 cells; doxorubicin; esculetin.
Figures
References
-
- Hrdina R, Gersl V, Klimtová I, Simůnek T, Machácková J, Adamcová M. Anthracycline-induced cardiotoxicity. Acta Medica (Hradec Kralove) 2000;43:75–82. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
