Engineering human ventricular heart muscles based on a highly efficient system for purification of human pluripotent stem cell-derived ventricular cardiomyocytes
- PMID: 28962583
- PMCID: PMC5622416
- DOI: 10.1186/s13287-017-0651-x
Engineering human ventricular heart muscles based on a highly efficient system for purification of human pluripotent stem cell-derived ventricular cardiomyocytes
Abstract
Background: Most infarctions occur in the left anterior descending coronary artery and cause myocardium damage of the left ventricle. Although current pluripotent stem cells (PSCs) and directed cardiac differentiation techniques are able to generate fetal-like human cardiomyocytes, isolation of pure ventricular cardiomyocytes has been challenging. For repairing ventricular damage, we aimed to establish a highly efficient purification system to obtain homogeneous ventricular cardiomyocytes and prepare engineered human ventricular heart muscles in a dish.
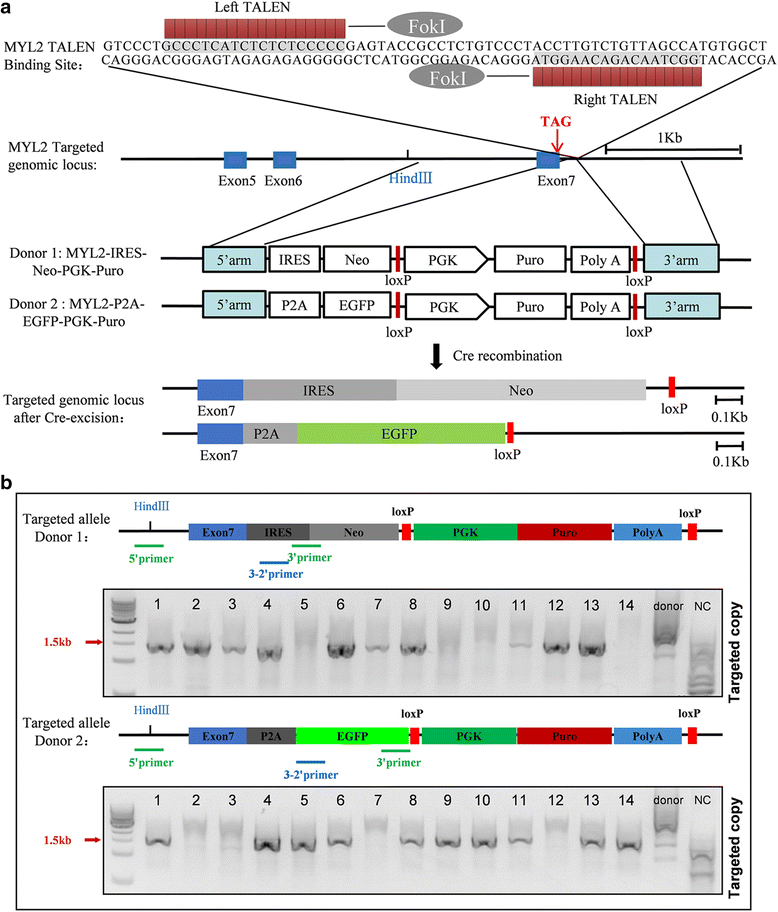
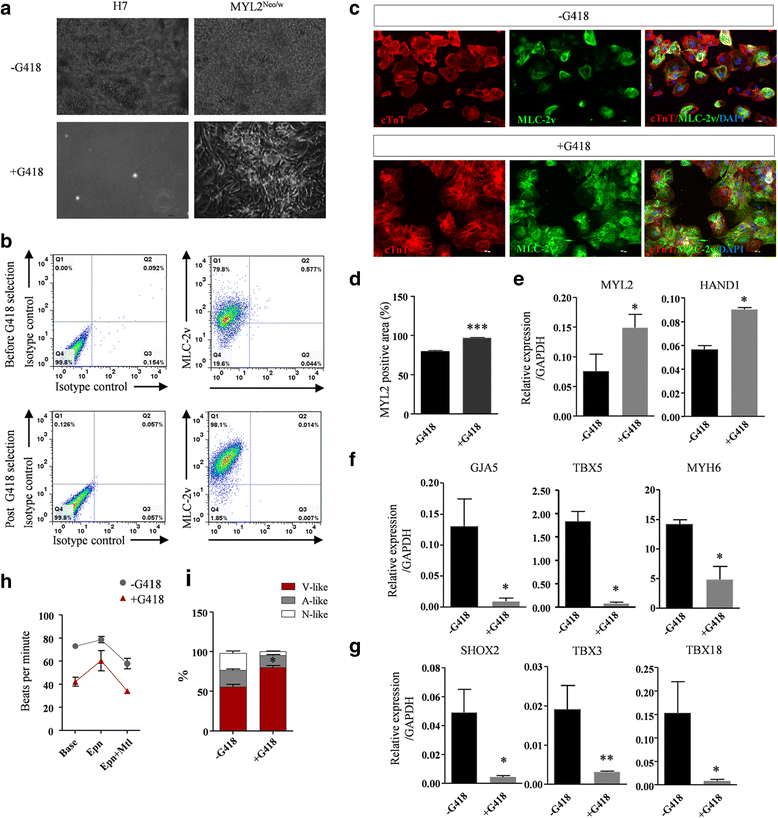
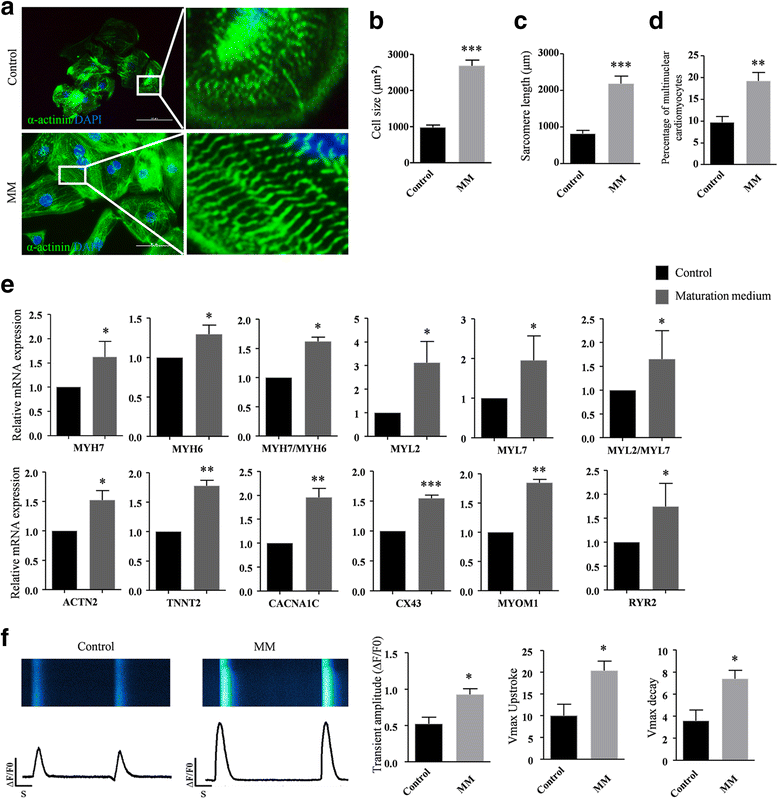
Methods: The purification system used TALEN-mediated genomic editing techniques to insert the neomycin or EGFP selection marker directly after the myosin light chain 2 (MYL2) locus in human pluripotent stem cells. Purified early ventricular cardiomyocytes were estimated by immunofluorescence, fluorescence-activated cell sorting, quantitative PCR, microelectrode array, and patch clamp. In subsequent experiments, the mixture of mature MYL2-positive ventricular cardiomyocytes and mesenchymal cells were cocultured with decellularized natural heart matrix. Histological and electrophysiology analyses of the formed tissues were performed 2 weeks later.
Results: Human ventricular cardiomyocytes were efficiently isolated based on the purification system using G418 or flow cytometry selection. When combined with the decellularized natural heart matrix as the scaffold, functional human ventricular heart muscles were prepared in a dish.
Conclusions: These engineered human ventricular muscles can be great tools for regenerative therapy of human ventricular damage as well as drug screening and ventricular-specific disease modeling in the future.
Keywords: Engineered human heart tissues; Engineered human ventricular heart muscles; Human pluripotent stem cells; Human ventricular cardiomyocytes; Myosin light chain 2; Myosin light chain 2v.
Conflict of interest statement
Ethics approval
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of the Fudan University (Committee approval number: 20140423) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). All human stem cells research followed the ISSCR Guidelines for the Conduct of Human Embryonic Stem Cell Research. The human ESC line H7 used in this study was obtained from WiCell Research Institute under specific Material Transfer Agreement. The human iPSC line was derived from human skin fibroblasts with informed consent approved previously by the Bioethics Committee of Zhongshan Hospital affiliated to Fudan University.
Consent for publication
All authors have contributed to, read, and approved the final manuscript for submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

