Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells
- PMID: 28962641
- PMCID: PMC5622468
- DOI: 10.1186/s13287-017-0659-2
Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells
Abstract
Background: Human dental mesenchymal stem cells (MSCs) are considered as highly accessible and attractive MSCs for use in regenerative medicine, yet some of their features are not as well characterized as other MSCs. Hypoxia-preconditioning and hypoxia-inducible factor 1 (HIF-1) alpha overexpression significantly improves MSC therapeutics, but the mechanisms involved are not fully understood. In the present study, we characterize immunomodulatory properties of dental MSCs and determine changes in their ability to modulate adaptive and innate immune populations after HIF-1 alpha overexpression.
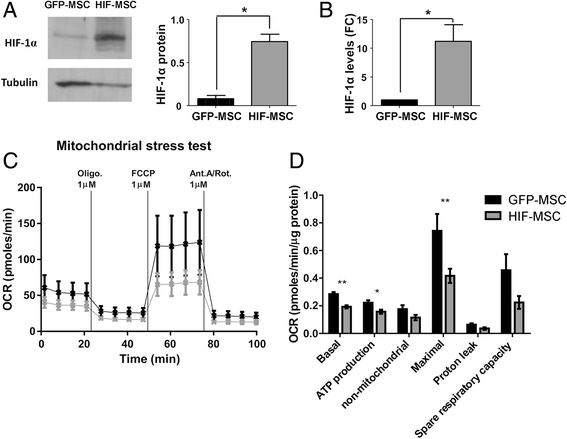
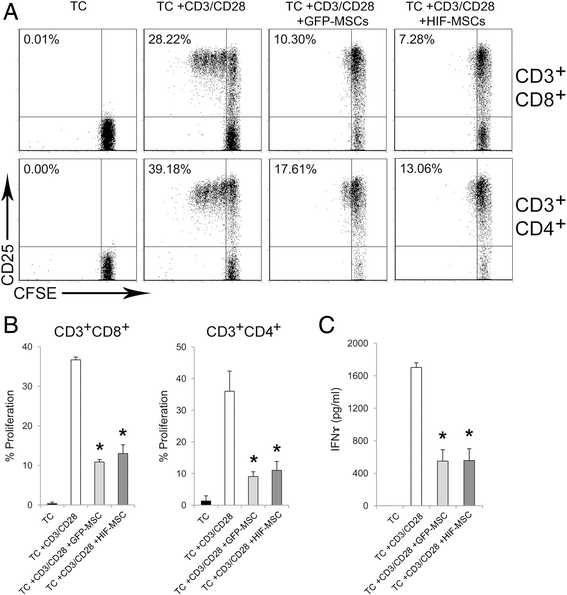
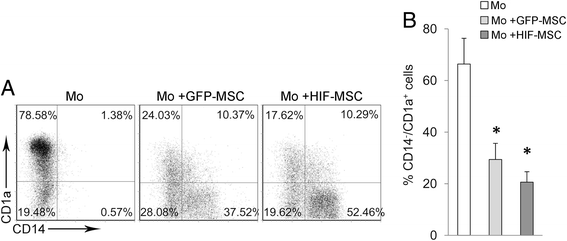
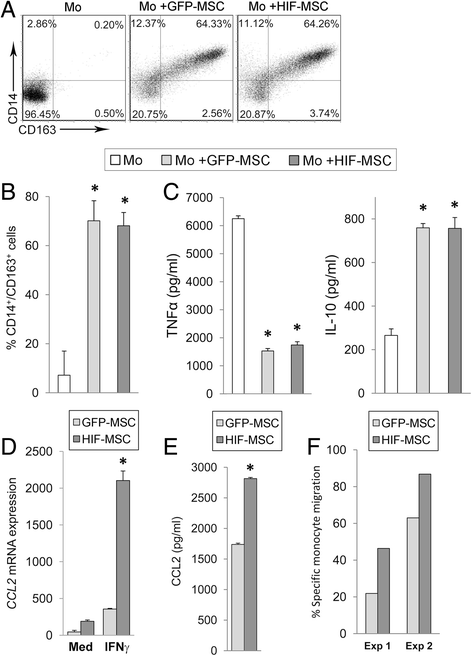
Methods: Human dental MSCs were stably transduced with green fluorescent protein (GFP-MSCs) or GFP-HIF-1 alpha lentivirus vectors (HIF-MSCs). A hypoxic-like metabolic profile was confirmed by mitochondrial and glycolysis stress test. Capacity of HIF-MSCs to modulate T-cell activation, dendritic cell differentiation, monocyte migration, and polarizations towards macrophages and natural killer (NK) cell lytic activity was assessed by a number of functional assays in co-cultures. The expression of relevant factors were determined by polymerase chain reaction (PCR) analysis and enzyme-linked immunosorbent assay (ELISA).
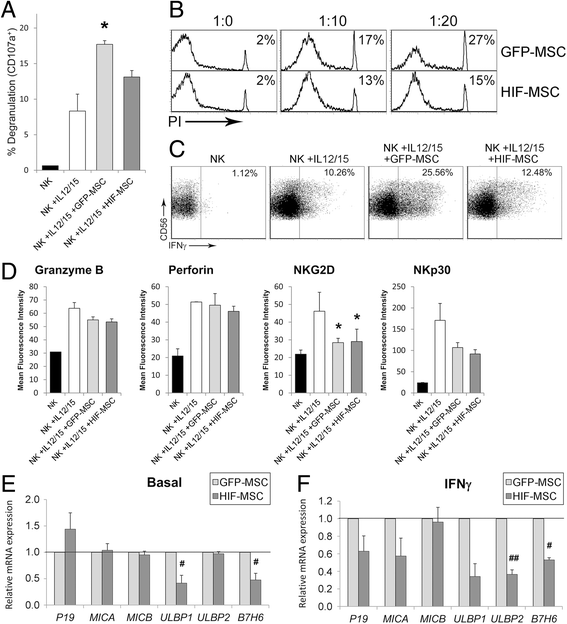
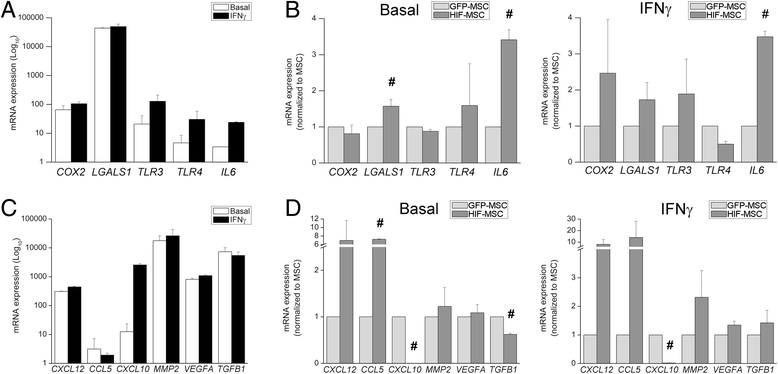
Results: While HIF-1 alpha overexpression did not modify the inhibition of T-cell activation by MSCs, HIF-MSCs impaired dendritic cell differentiation more efficiently. In addition, HIF-MSCs showed a tendency to induce higher attraction of monocytes, which differentiate into suppressor macrophages, and exhibited enhanced resistance to NK cell-mediated lysis, which supports the improved therapeutic capacity of HIF-MSCs. HIF-MSCs also displayed a pro-angiogenic profile characterized by increased expression of CXCL12/SDF1 and CCL5/RANTES and complete loss of CXCL10/IP10 transcription.
Conclusions: Immunomodulation and expression of trophic factors by dental MSCs make them perfect candidates for cell therapy. Overexpression of HIF-1 alpha enhances these features and increases their resistance to allogenic NK cell lysis and, hence, their potential in vivo lifespan. Our results further support the use of HIF-1 alpha-expressing dental MSCs for cell therapy in tissue injury and immune disorders.
Keywords: Cell therapy; Dental pulp; Hypoxia-inducible factor 1 alpha subunit (HIF-1 alpha); Immunomodulation; Mesenchymal stem cells (MSCs).
Conflict of interest statement
Ethics approval and consent to participate
All procedures were approved by the Instituto de Salud Carlos III and Institutional Ethical and Animal Care Committees. Buffy coats of healthy donors were obtained after informed consent (Centro de Transfusión de la Comunidad de Madrid, Spain).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes.Stem Cells. 2017 Jul;35(7):1747-1759. doi: 10.1002/stem.2618. Epub 2017 Apr 24. Stem Cells. 2017. PMID: 28376567
-
LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning.Stem Cell Res Ther. 2018 Oct 25;9(1):280. doi: 10.1186/s13287-018-1031-x. Stem Cell Res Ther. 2018. PMID: 30359325 Free PMC article.
-
HIF-1α and Pro-Inflammatory Signaling Improves the Immunomodulatory Activity of MSC-Derived Extracellular Vesicles.Int J Mol Sci. 2021 Mar 26;22(7):3416. doi: 10.3390/ijms22073416. Int J Mol Sci. 2021. PMID: 33810359 Free PMC article.
-
Immunomodulatory properties of dental tissue-derived mesenchymal stem cells.Oral Dis. 2014 Jan;20(1):25-34. doi: 10.1111/odi.12086. Epub 2013 Mar 6. Oral Dis. 2014. PMID: 23463961 Review.
-
Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells.Cytotherapy. 2016 Feb;18(2):160-71. doi: 10.1016/j.jcyt.2015.10.011. Cytotherapy. 2016. PMID: 26794710 Review.
Cited by
-
Mircrining the injured heart with stem cell-derived exosomes: an emerging strategy of cell-free therapy.Stem Cell Res Ther. 2020 Jan 9;11(1):23. doi: 10.1186/s13287-019-1548-7. Stem Cell Res Ther. 2020. PMID: 31918755 Free PMC article. Review.
-
Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration.Front Immunol. 2019 Apr 2;10:663. doi: 10.3389/fimmu.2019.00663. eCollection 2019. Front Immunol. 2019. PMID: 31001270 Free PMC article. Review.
-
Rebuilding the myocardial microenvironment to enhance mesenchymal stem cells-mediated regeneration in ischemic heart disease.Front Bioeng Biotechnol. 2024 Sep 20;12:1468833. doi: 10.3389/fbioe.2024.1468833. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39372432 Free PMC article. Review.
-
Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential.Int J Mol Sci. 2024 Feb 6;25(4):1986. doi: 10.3390/ijms25041986. Int J Mol Sci. 2024. PMID: 38396665 Free PMC article. Review.
-
A New Target of Dental Pulp-Derived Stem Cell-Based Therapy on Recipient Bone Marrow Niche in Systemic Lupus Erythematosus.Int J Mol Sci. 2022 Mar 23;23(7):3479. doi: 10.3390/ijms23073479. Int J Mol Sci. 2022. PMID: 35408840 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

