Construction of ultrasonic nanobubbles carrying CAIX polypeptides to target carcinoma cells derived from various organs
- PMID: 28962657
- PMCID: PMC5622542
- DOI: 10.1186/s12951-017-0307-0
Construction of ultrasonic nanobubbles carrying CAIX polypeptides to target carcinoma cells derived from various organs
Abstract
Background: Ultrasound molecular imaging is a novel diagnostic approach for tumors, whose key link is the construction of targeted ultrasound contrast agents. However, available targeted ultrasound contrast agents for molecular imaging of tumors are only achieving imaging in blood pool or one type tumor. No targeted ultrasound contrast agents have realized targeted ultrasound molecular imaging of tumor parenchymal cells in a variety of solid tumors so far. Carbonic anhydrase IX (CAIX) is highly expressed on cell membranes of various malignant solid tumors, so it's a good target for ultrasound molecular imaging. Here, targeted nanobubbles carrying CAIX polypeptides for targeted binding to a variety of malignant tumors were constructed, and targeted binding ability and ultrasound imaging effect in different types of tumors were evaluated.
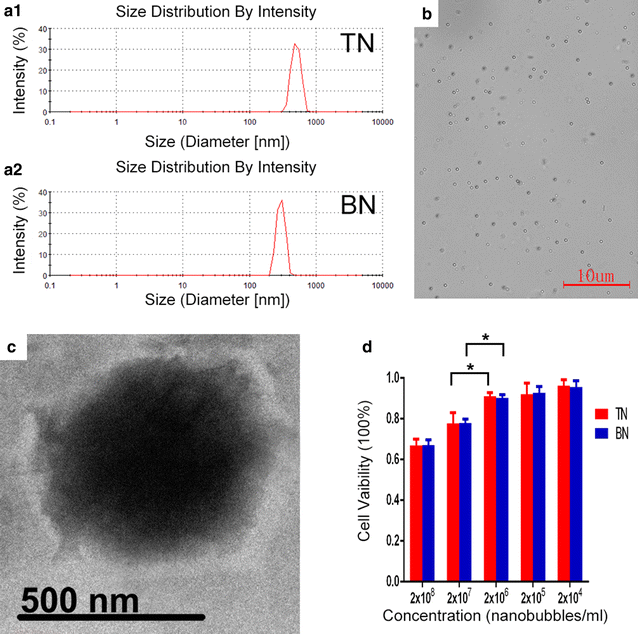
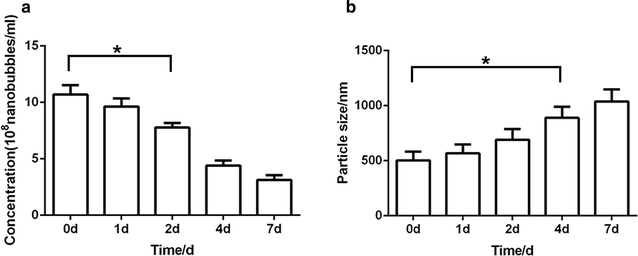
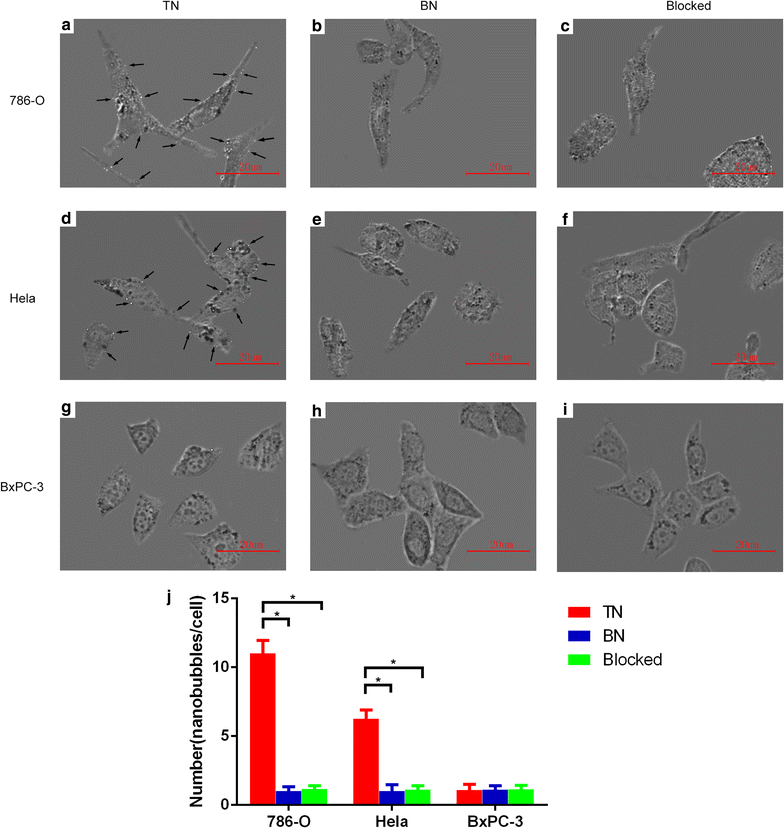
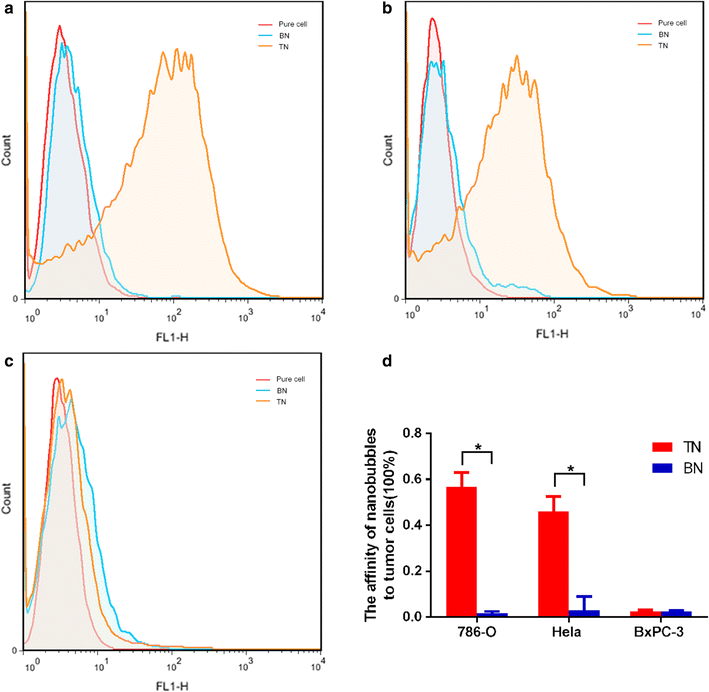
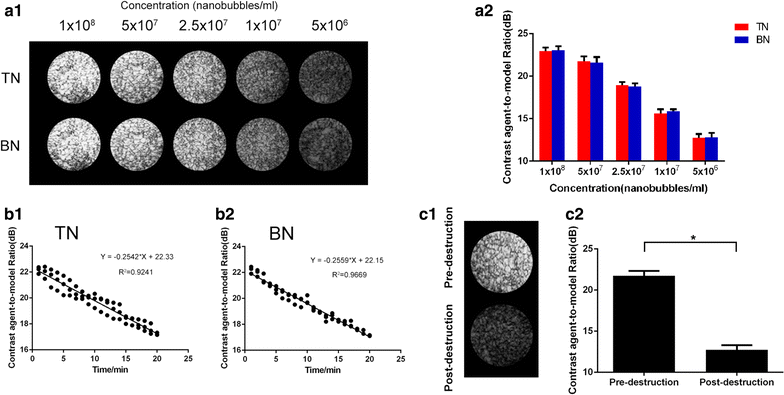
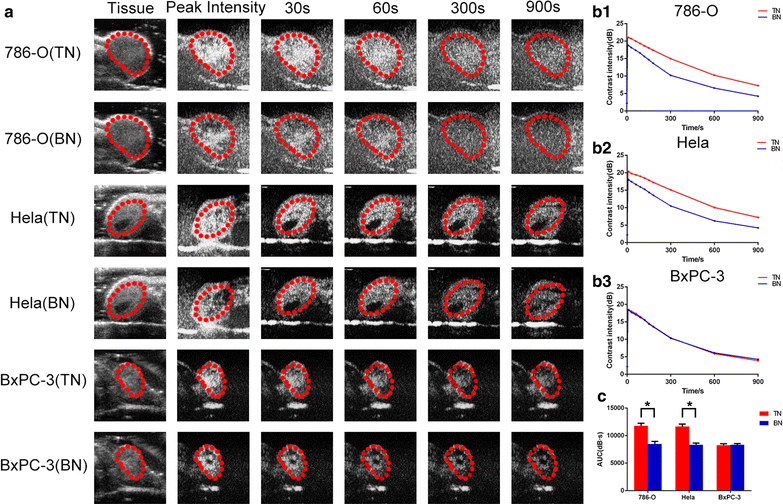
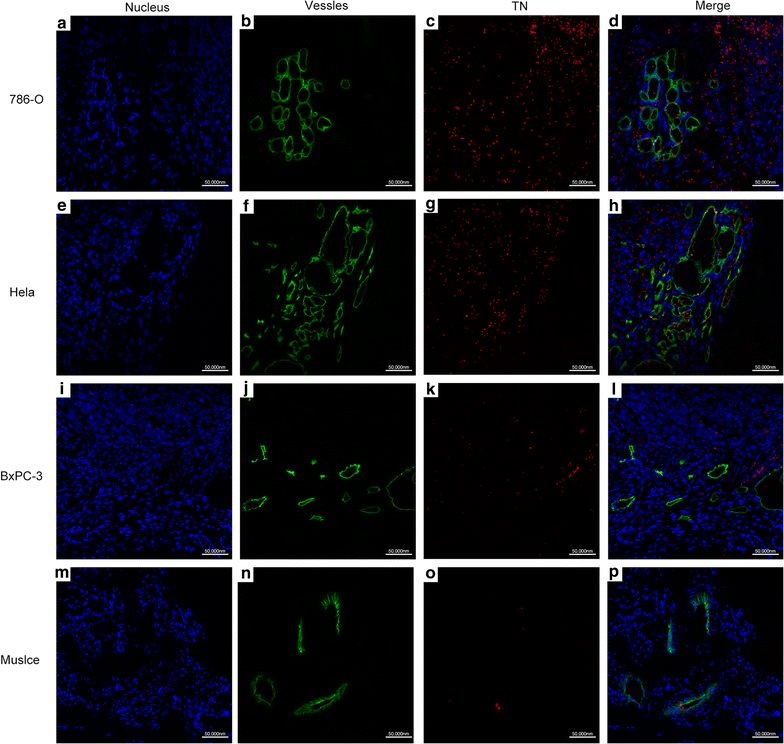
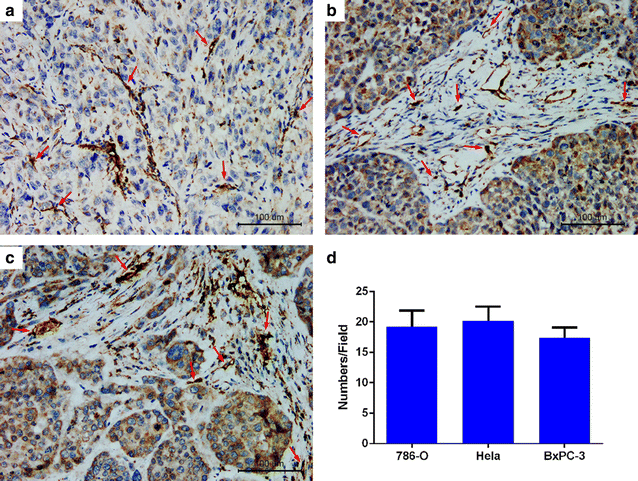
Results: The mean diameter of lipid targeted nanobubbles was (503.7 ± 78.47) nm, and the polypeptides evenly distributed on the surfaces of targeted nanobubbles, which possessed the advantages of homogenous particle size, high stability, and good safety. Targeted nanobubbles could gather around CAIX-positive cells (786-O and Hela cells), while they cannot gather around CAIX-negative cells (BxPC-3 cells) in vitro, and the affinity of targeted nanobubbles to CAIX-positive cells were significantly higher than that to CAIX-negative cells (P < 0.05). Peak intensity and duration time of targeted nanobubbles and blank nanobubbles were different in CAIX-positive transplanted tumor tissues in vivo (P < 0.05). Moreover, targeted nanobubbles in CAIX-positive transplanted tumor tissues produced higher peak intensity and longer duration time than those in CAIX-negative transplanted tumor tissues (P < 0.05). Finally, immunofluorescence not only confirmed targeted nanobubbles could pass through blood vessels to enter in tumor tissue spaces, but also clarified imaging differences of targeted nanobubbles in different types of transplanted tumor tissues.
Conclusions: Targeted nanobubbles carrying CAIX polypeptides can specifically enhance ultrasound imaging in CAIX-positive transplanted tumor tissues and could potentially be used in early diagnosis of a variety of solid tumors derived from various organs.
Keywords: Carbonic anhydrase IX; Malignant tumors; Targeted nanobubbles; Ultrasound molecular imaging.
Figures

Similar articles
-
CAIX aptamer-functionalized targeted nanobubbles for ultrasound molecular imaging of various tumors.Int J Nanomedicine. 2018 Oct 16;13:6481-6495. doi: 10.2147/IJN.S176287. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30410333 Free PMC article.
-
Indocyanine Green-Loaded Nanobubbles Targeting Carbonic Anhydrase IX for Multimodal Imaging of Renal Cell Carcinoma.Int J Nanomedicine. 2023 May 23;18:2757-2776. doi: 10.2147/IJN.S408977. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37250472 Free PMC article.
-
Anti-G250 nanobody-functionalized nanobubbles targeting renal cell carcinoma cells for ultrasound molecular imaging.Nanotechnology. 2020 May 15;31(20):205101. doi: 10.1088/1361-6528/ab7040. Epub 2020 Feb 27. Nanotechnology. 2020. PMID: 32107342
-
Targeting carbonic anhydrase IX with small organic ligands.Curr Opin Chem Biol. 2015 Jun;26:48-54. doi: 10.1016/j.cbpa.2015.02.005. Epub 2015 Feb 24. Curr Opin Chem Biol. 2015. PMID: 25721398 Review.
-
New Developments in Carbonic Anhydrase IX-Targeted Fluorescence and Nuclear Imaging Agents.Int J Mol Sci. 2022 May 30;23(11):6125. doi: 10.3390/ijms23116125. Int J Mol Sci. 2022. PMID: 35682802 Free PMC article. Review.
Cited by
-
Nanobubble Contrast Enhanced Ultrasound Imaging: A Review.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024 Nov-Dec;16(6):e2007. doi: 10.1002/wnan.2007. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024. PMID: 39511794 Free PMC article. Review.
-
G250 Antigen-Targeting Drug-Loaded Nanobubbles Combined with Ultrasound Targeted Nanobubble Destruction: A Potential Novel Treatment for Renal Cell Carcinoma.Int J Nanomedicine. 2020 Jan 8;15:81-95. doi: 10.2147/IJN.S230879. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32021166 Free PMC article.
-
Targeted Nanobubbles Carrying Indocyanine Green for Ultrasound, Photoacoustic and Fluorescence Imaging of Prostate Cancer.Int J Nanomedicine. 2020 Jun 17;15:4289-4309. doi: 10.2147/IJN.S243548. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32606678 Free PMC article.
-
"PFH/AGM-CBA/HSV-TK/LIPOSOME-Affibody": Novel Targeted Nano Ultrasound Contrast Agents for Ultrasound Imaging and Inhibited the Growth of ErbB2-Overexpressing Gastric Cancer Cells.Drug Des Devel Ther. 2022 May 18;16:1515-1530. doi: 10.2147/DDDT.S351623. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35611358 Free PMC article.
-
Sink or float? Characterization of shell-stabilized bulk nanobubbles using a resonant mass measurement technique.Nanoscale. 2019 Jan 17;11(3):851-855. doi: 10.1039/c8nr08763f. Nanoscale. 2019. PMID: 30601524 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

