Shared decision making for stroke prevention in atrial fibrillation: study protocol for a randomized controlled trial
- PMID: 28962662
- PMCID: PMC5622521
- DOI: 10.1186/s13063-017-2178-y
Shared decision making for stroke prevention in atrial fibrillation: study protocol for a randomized controlled trial
Abstract
Background: Nonvalvular atrial fibrillation (AF) is a common ongoing health problem that places patients at risk of stroke. Whether and how a patient addresses this risk depends on each patient's goals, context, and values. Consequently, leading cardiovascular societies recommend using shared decision making (SDM) to individualize antithrombotic treatment in patients with AF. The aim of this study is to assess the extent to which the ANTICOAGULATION CHOICE conversation tool promotes high-quality SDM and influences anticoagulation uptake and adherence in patients with AF at risk of strokes.
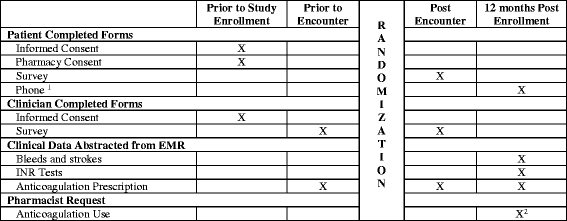
Methods: This study protocol describes a multicenter, encounter-level, randomized trial to assess the effect of using the ANTICOAGULATION CHOICE conversation tool in the clinical encounter, compared to usual care. The participating centers include an academic hospital system, a suburban community group practice, and an urban safety net hospital, all in Minnesota, USA. Patients with ongoing nonvalvular AF at risk of strokes (CHA2DS2-VASc score ≥ 1 in men, or ≥ 2 in women) will be eligible for participation. We aim to include 999 patients and their clinicians. The primary outcome is the quality of SDM as perceived by participants, and as assessed by a post-encounter survey that ascertains (a) knowledge transfer, (b) concordance of the decision made, (c) quality of communication, and (d) satisfaction with the decision-making process. Recordings of encounters will be reviewed to assess the extent of patient involvement and how participants use the tool (fidelity). Anticoagulant use, choice of agent, and adherence will be drawn from patients' medical and pharmacy records. Strokes and bleeding events will be drawn from patient records.
Discussion: This study will provide a valid and precise measure of the effect of the ANTICOAGULATION CHOICE conversation tool on SDM quality and processes, and on the treatment choices and adherence to therapy among AF patients at risk of stroke.
Trial registration: ClinicalTrials.gov, NCT02905032 . Registered on 9 September 2016.
Keywords: Anticoagulation; Atrial fibrillation; Conversation aid; Decision aid; Medication adherence; Medication uptake; Shared decision making; communication.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
Institutional Review Board approval has been obtained from the lead coordinating center, the Mayo Clinic (approval number 16-005409) and from the two participating external sites. All participating patients and clinicians must provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effect of Shared Decision-Making for Stroke Prevention on Treatment Adherence and Safety Outcomes in Patients With Atrial Fibrillation: A Randomized Clinical Trial.J Am Heart Assoc. 2022 Jan 18;11(2):e023048. doi: 10.1161/JAHA.121.023048. Epub 2022 Jan 13. J Am Heart Assoc. 2022. PMID: 35023356 Free PMC article. Clinical Trial.
-
Assessment of Shared Decision-making for Stroke Prevention in Patients With Atrial Fibrillation: A Randomized Clinical Trial.JAMA Intern Med. 2020 Sep 1;180(9):1215-1224. doi: 10.1001/jamainternmed.2020.2908. JAMA Intern Med. 2020. PMID: 32897386 Free PMC article. Clinical Trial.
-
Normalization of a conversation tool to promote shared decision making about anticoagulation in patients with atrial fibrillation within a practical randomized trial of its effectiveness: a cross-sectional study.Trials. 2020 May 12;21(1):395. doi: 10.1186/s13063-020-04305-2. Trials. 2020. PMID: 32398149 Free PMC article. Clinical Trial.
-
Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation.Tex Heart Inst J. 2015 Feb 1;42(1):6-15. doi: 10.14503/THIJ-14-4353. eCollection 2015 Feb. Tex Heart Inst J. 2015. PMID: 25873792 Free PMC article. Review.
-
Decision aids for shared decision-making and appropriate anticoagulation therapy in patients with atrial fibrillation: a systematic review and meta-analysis.Eur J Cardiovasc Nurs. 2022 Mar 3;21(2):97-106. doi: 10.1093/eurjcn/zvab085. Eur J Cardiovasc Nurs. 2022. PMID: 34550376
Cited by
-
Cost Conversations About Anticoagulation Between Patients With Atrial Fibrillation and Their Clinicians: A Secondary Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2021 Jul 1;4(7):e2116009. doi: 10.1001/jamanetworkopen.2021.16009. JAMA Netw Open. 2021. PMID: 34255051 Free PMC article. Clinical Trial.
-
Using Patient Decision Aids for Cardiology Care in Diverse Populations.Curr Cardiol Rep. 2023 Nov;25(11):1543-1553. doi: 10.1007/s11886-023-01953-z. Epub 2023 Nov 9. Curr Cardiol Rep. 2023. PMID: 37943426 Free PMC article. Review.
-
Developing a Conversation Aid to Support Shared Decision Making: Reflections on Designing Anticoagulation Choice.Mayo Clin Proc. 2019 Apr;94(4):686-696. doi: 10.1016/j.mayocp.2018.08.030. Epub 2019 Jan 11. Mayo Clin Proc. 2019. PMID: 30642640 Free PMC article. Review.
-
Effect of Shared Decision-Making for Stroke Prevention on Treatment Adherence and Safety Outcomes in Patients With Atrial Fibrillation: A Randomized Clinical Trial.J Am Heart Assoc. 2022 Jan 18;11(2):e023048. doi: 10.1161/JAHA.121.023048. Epub 2022 Jan 13. J Am Heart Assoc. 2022. PMID: 35023356 Free PMC article. Clinical Trial.
-
Factors Associated With Patient Engagement in Shared Decision-Making for Stroke Prevention Among Older Adults with Atrial Fibrillation.Can Geriatr J. 2021 Sep 1;24(3):174-183. doi: 10.5770/cgj.24.475. eCollection 2021 Sep. Can Geriatr J. 2021. PMID: 34484500 Free PMC article.
References
-
- Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, Spolveri S, Baruffi MC, Landini G, Ghetti A, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project) Stroke. 2001;32(2):392–8. doi: 10.1161/01.STR.32.2.392. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

