Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers
- PMID: 28963439
- PMCID: PMC5620371
- DOI: 10.1091/mbc.E17-06-0416
Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers
Abstract
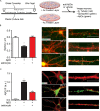
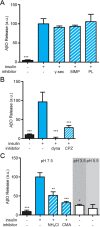
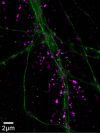
Synaptopathy underlying memory deficits in Alzheimer's disease (AD) is increasingly thought to be instigated by toxic oligomers of the amyloid beta peptide (AβOs). Given the long latency and incomplete penetrance of AD dementia with respect to Aβ pathology, we hypothesized that factors present in the CNS may physiologically protect neurons from the deleterious impact of AβOs. Here we employed physically separated neuron-astrocyte cocultures to investigate potential non-cell autonomous neuroprotective factors influencing AβO toxicity. Neurons cultivated in the absence of an astrocyte feeder layer showed abundant AβO binding to dendritic processes and associated synapse deterioration. In contrast, neurons in the presence of astrocytes showed markedly reduced AβO binding and synaptopathy. Results identified the protective factors released by astrocytes as insulin and insulin-like growth factor-1 (IGF1). The protective mechanism involved release of newly bound AβOs into the extracellular medium dependent upon trafficking that was sensitive to exosome pathway inhibitors. Delaying insulin treatment led to AβO binding that was no longer releasable. The neuroprotective potential of astrocytes was itself sensitive to chronic AβO exposure, which reduced insulin/IGF1 expression. Our findings support the idea that physiological protection against synaptotoxic AβOs can be mediated by astrocyte-derived insulin/IGF1, but that this protection itself is vulnerable to AβO buildup.
© 2017 Pitt et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures





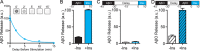


References
-
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. - PubMed
-
- Aoki N, Jin-no S, Nakagawa Y, Asai N, Arakawa E, Tamura N, Tamura T, Matsuda T. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology. 2007;148:3850–3862. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

