Foxp3+ Tregs are recruited to the retina to repair pathological angiogenesis
- PMID: 28963474
- PMCID: PMC5622066
- DOI: 10.1038/s41467-017-00751-w
Foxp3+ Tregs are recruited to the retina to repair pathological angiogenesis
Abstract
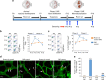
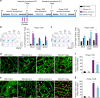
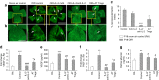
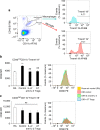
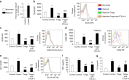
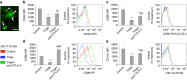
Neovascular retinopathies are major causes of vision loss; yet treatments to prevent the condition are inadequate. The role of regulatory T cells in neovascular retinopathy is unknown. Here we show that in retinopathy regulatory T cells are transiently increased in lymphoid organs and the retina, but decline when neovascularization is established. The decline is prevented following regulatory T cells expansion with an IL-2/anti-IL-2 mAb complex or the adoptive transfer of regulatory T cells. Further, both approaches reduce vasculopathy (vaso-obliteration, neovascularization, vascular leakage) and alter the activation of Tmem119+ retinal microglia. Our in vitro studies complement these findings, showing that retinal microglia co-cultured with regulatory T cells exhibit a reduction in co-stimulatory molecules and pro-inflammatory mediators that is attenuated by CTLA-4 blockade. Collectively, we demonstrate that regulatory T cells are recruited to the retina and, when expanded in number, repair the vasculature. Manipulation of regulatory T cell numbers is a previously unrecognized, and promising avenue for therapies to prevent blinding neovascular retinopathies.The local immune responses in the eye are attenuated to preserve sight. Surprisingly, Deliyanti et al. show that regulatory T cells (Tregs) take an active role in protecting the eye from neovascularization in oxygen-induced retinopathy, and that interventions that augment the retinal Treg numbers reduce neovascular retinopathy in mice.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Tregs that express the Foxp3 transcription factor can influence oxygen-induced retinopathy.Surv Ophthalmol. 2018 May-Jun;63(3):446. doi: 10.1016/j.survophthal.2017.12.005. Epub 2017 Dec 15. Surv Ophthalmol. 2018. PMID: 29248531 No abstract available.
References
-
- Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Med. J. Aust. 1951;2:48–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

