Human Amniotic Membrane Mesenchymal Stem Cells inhibit Neutrophil Extracellular Traps through TSG-6
- PMID: 28963485
- PMCID: PMC5622031
- DOI: 10.1038/s41598-017-10962-2
Human Amniotic Membrane Mesenchymal Stem Cells inhibit Neutrophil Extracellular Traps through TSG-6
Abstract
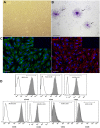
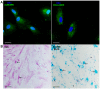
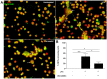
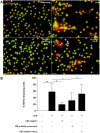
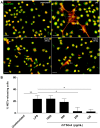
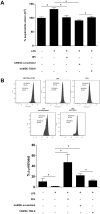
The mesenchymal stem cells obtained from human amniotic membrane (hAMSC) possess immunosuppressive functions through soluble factors such as prostanoids and proteins; thus, they have been proposed to ameliorate inflammatory processes. On the other hand, activated neutrophils are cells of the first line of immune defense that are able to release extracellular traps (NETs). NETs are formed of DNA and granular components; however, the excessive release of NETs is associated with the development of autoimmune and chronic inflammatory diseases. In this study, we identified that conditioned medium (CM) from hAMSC was able to diminish NETs release, as well as the production of reactive oxygen species (ROS) and the mitochondrial membrane potential from LPS-stimulated mouse bone marrow-derived neutrophils (BMN). Interestingly, NETs inhibition, ROS levels decrease and mitochondrial membrane potential loss were reverted when LPS-stimulated murine derived BMN were exposed to the CM from hAMSC transfected with TSG-6-siRNA. Finally, rhTSG6 was able to significantly diminish NETs release in BMN. These data suggest an inhibition mechanism of NETs ROS-dependent in which TSG-6 participates. Consequently, we propose the hAMSC use as a therapeutic candidate in the treatment of inflammatory diseases in which NETs are involved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
ONO-5046 suppresses reactive oxidative species-associated formation of neutrophil extracellular traps.Life Sci. 2018 Oct 1;210:243-250. doi: 10.1016/j.lfs.2018.09.008. Epub 2018 Sep 6. Life Sci. 2018. PMID: 30195031
-
IL-17A expressed on neutrophil extracellular traps promotes mesenchymal stem cell differentiation toward bone-forming cells in ankylosing spondylitis.Eur J Immunol. 2021 Apr;51(4):930-942. doi: 10.1002/eji.202048878. Epub 2021 Jan 22. Eur J Immunol. 2021. PMID: 33340091
-
IRF-1 Intervention in the Classical ROS-Dependent Release of NETs during LPS-Induced Acute Lung Injury in Mice.Inflammation. 2019 Feb;42(1):387-403. doi: 10.1007/s10753-018-0903-7. Inflammation. 2019. PMID: 30315525
-
Neutrophils' Extracellular Trap Mechanisms: From Physiology to Pathology.Int J Mol Sci. 2022 Oct 25;23(21):12855. doi: 10.3390/ijms232112855. Int J Mol Sci. 2022. PMID: 36361646 Free PMC article. Review.
-
Role of Neutrophil Extracellular Traps in Asthma and Chronic Obstructive Pulmonary Disease.Chin Med J (Engl). 2017 Mar 20;130(6):730-736. doi: 10.4103/0366-6999.201608. Chin Med J (Engl). 2017. PMID: 28303858 Free PMC article. Review.
Cited by
-
Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine.Int J Mol Sci. 2019 Mar 29;20(7):1573. doi: 10.3390/ijms20071573. Int J Mol Sci. 2019. PMID: 30934825 Free PMC article. Review.
-
Tafazzin deficiency in mouse mesenchymal stem cells promote reprogramming of activated B lymphocytes toward immunosuppressive phenotypes.FASEB J. 2022 Aug;36(8):e22443. doi: 10.1096/fj.202200145R. FASEB J. 2022. PMID: 35816277 Free PMC article.
-
Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury.Int J Mol Sci. 2020 Sep 15;21(18):6761. doi: 10.3390/ijms21186761. Int J Mol Sci. 2020. PMID: 32942629 Free PMC article. Review.
-
Human Amniotic Membrane Mesenchymal Stem Cell-Synthesized PGE2 Exerts an Immunomodulatory Effect on Neutrophil Extracellular Trap in a PAD-4-Dependent Pathway through EP2 and EP4.Cells. 2022 Sep 10;11(18):2831. doi: 10.3390/cells11182831. Cells. 2022. PMID: 36139406 Free PMC article.
-
A comparison of isolation and culture protocols for human amniotic mesenchymal stem cells.Cell Cycle. 2022 Aug;21(15):1543-1556. doi: 10.1080/15384101.2022.2060641. Epub 2022 Apr 12. Cell Cycle. 2022. PMID: 35412950 Free PMC article.
References
-
- Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

