Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation
- PMID: 28963531
- PMCID: PMC5622101
- DOI: 10.1038/s41598-017-12635-6
Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation
Abstract
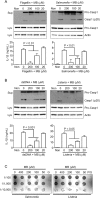
Methylene blue (MB), which has antioxidant, anti-inflammatory, neuroprotective, and mitochondria protective effects, has been widely used as a dye and medication. However, the effect of MB on inflammasome activation has not yet been studied. Inflammasomes are multi-protein complexes that induce maturation of interleukins (ILs)-1β and -18 as well as caspase-1-mediated cell death, known as pyroptosis. Dysregulation of inflammasomes causes several diseases such as type 2 diabetes, Alzheimer's disease, and gout. In this study, we assess the effect of MB on inflammasome activation in macrophages. As the result, MB attenuated activation of canonical inflammasomes such as NLRP3, NLRC4, and AIM2 as well as non-canonical inflammasome activation. In addition, MB inhibited upstream signals such as inflammasome assembly, phagocytosis, and gene expression of inflammasome components via inhibition of NF-κB signaling. Furthermore, MB reduced the activity of caspase-1. The anti-inflammasome properties of MB were further confirmed in mice models. Thus, we suggest that MB is a broad-spectrum anti-inflammasome candidate molecule.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

