Measuring ex vivo drug susceptibility in Plasmodium vivax isolates from Cambodia
- PMID: 28964258
- PMCID: PMC5622433
- DOI: 10.1186/s12936-017-2034-2
Measuring ex vivo drug susceptibility in Plasmodium vivax isolates from Cambodia
Abstract
Background: While intensive Plasmodium falciparum multidrug resistance surveillance continues in Cambodia, relatively little is known about Plasmodium vivax drug resistance in Cambodia or elsewhere. To investigate P. vivax anti-malarial susceptibility in Cambodia, 76 fresh P. vivax isolates collected from Oddar Meanchey (northern Cambodia) in 2013-2015 were assessed for ex vivo drug susceptibility using the microscopy-based schizont maturation test (SMT) and a Plasmodium pan-species lactate dehydrogenase (pLDH) ELISA. P. vivax multidrug resistance gene 1 (pvmdr1) mutations, and copy number were analysed in a subset of isolates.
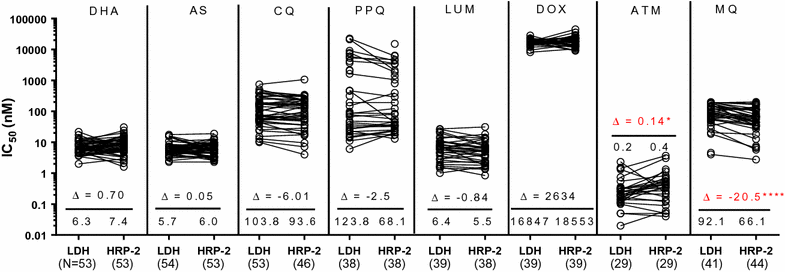
Results: Ex vivo testing was interpretable in 80% of isolates using the pLDH-ELISA, but only 25% with the SMT. Plasmodium vivax drug susceptibility by pLDH-ELISA was directly compared with 58 P. falciparum isolates collected from the same locations in 2013-4, tested by histidine-rich protein-2 ELISA. Median pLDH-ELISA IC50 of P. vivax isolates was significantly lower for dihydroartemisinin (3.4 vs 6.3 nM), artesunate (3.2 vs 5.7 nM), and chloroquine (22.1 vs 103.8 nM) than P. falciparum but higher for mefloquine (92 vs 66 nM). There were not significant differences for lumefantrine or doxycycline. Both P. vivax and P. falciparum had comparable median piperaquine IC50 (106.5 vs 123.8 nM), but some P. falciparum isolates were able to grow in much higher concentrations above the normal standard range used, attaining up to 100-fold greater IC50s than P. vivax. A high percentage of P. vivax isolates had pvmdr1 Y976F (78%) and F1076L (83%) mutations but none had pvmdr1 amplification.
Conclusion: The findings of high P. vivax IC50 to mefloquine and piperaquine, but not chloroquine, suggest significant drug pressure from drugs used to treat multidrug resistant P. falciparum in Cambodia. Plasmodium vivax isolates are frequently exposed to mefloquine and piperaquine due to mixed infections and the long elimination half-life of these drugs. Difficulty distinguishing infection due to relapsing hypnozoites versus blood-stage recrudescence complicates clinical detection of P. vivax resistance, while well-validated molecular markers of chloroquine resistance remain elusive. The pLDH assay may be a useful adjunctive tool for monitoring for emerging drug resistance, though more thorough validation is needed. Given high grade clinical chloroquine resistance observed recently in neighbouring countries, low chloroquine IC50 values seen here should not be interpreted as susceptibility in the absence of clinical data. Incorporating pLDH monitoring with therapeutic efficacy studies for individuals with P. vivax will help to further validate this field-expedient method.
Keywords: Cambodia; Drug resistance; Ex vivo assay; Plasmodium vivax; pvmdr1.
Figures



References
-
- Wongsrichanalai C, Sirichaisinthop J, Karwacki JJ, Congpuong K, Miller RS, Pang L, et al. Drug resistant malaria on the Thai-Myanmar and Thai-Cambodian borders. Southeast Asian J Trop Med Public Health. 2001;32:41–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

