Preferences of Patients and Pharmacists with Regard to the Management of Drug-Drug Interactions: A Choice-Based Conjoint Analysis
- PMID: 28965265
- PMCID: PMC5808046
- DOI: 10.1007/s40264-017-0601-7
Preferences of Patients and Pharmacists with Regard to the Management of Drug-Drug Interactions: A Choice-Based Conjoint Analysis
Abstract
Introduction: The management of drug-drug interactions (DDIs) is a complex process in which risk-benefit assessments should be combined with the patient's perspective.
Objective: The aim of this study was to determine patients' and pharmacists' preferences regarding DDI management.
Methods: We conducted a choice-based conjoint survey about a fictitious DDI concerning the combination of a cardiovascular drug and an antibiotic for pneumonia. Patients and pharmacists had to choose 12 times between two management options. The options were described by five attributes, including risk, benefit and practical consequences. Each attribute could have two different levels, which were varied over the choice tasks. Latent class analysis was used to identify potential classes of respondents with distinct patterns of similar preferences.
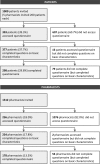
Results: In total, 298 patients and 178 pharmacists completed the questionnaire. The latent class model for both patients and pharmacists resulted in three classes. For patients, in one class the most importance was attached to avoiding switch of medication (class probability 20%), in a second class to fewer adverse events (41%), and in a third class to blood sampling (39%). For pharmacists, again one class attached the highest importance to avoiding switch of medication (31%). The other classes gave priority to curing pneumonia (31%) and avoiding blood sampling (38%).
Conclusion: The results showed diverging preferences regarding DDI management among both patients and pharmacists. Different groups attached different value to risk and benefit versus practical considerations. Awareness of existing variability in preferences among and between pharmacists and patients is a step towards shared decision making in DDI management.
Conflict of interest statement
Funding
MH received an unconditional grant from Sawtooth Software for using Sawtooth Software Lighthouse Studio and Sawtooth Software Hosting. This research received no other specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
Mette Heringa, Annemieke Floor-Schreudering, Hans Wouters, Peter A.G.M. De Smet and Marcel L. Bouvy have no conflicts of interest that are directly relevant to the content of this study.
Human Subjects
The Institutional Review Board of the Division of Pharmacoepidemiology and Clinical Pharmacology of Utrecht University approved the investigation, and the work was conducted in compliance with its requirements. Only anonymous data were collected. Respondents did not sign informed consent, as an anonymous survey among volunteers did not fall within the scope of the Dutch Act on Medical Research Involving Human Subjects.
Figures

Similar articles
-
Aspects influencing patients' preferences for the management of drug-drug interactions: A focus group study.Patient Educ Couns. 2018 Apr;101(4):723-729. doi: 10.1016/j.pec.2017.11.010. Epub 2017 Nov 22. Patient Educ Couns. 2018. PMID: 29173959
-
Pharmacists' awareness of clinical decision support in pharmacy information systems: an exploratory evaluation.Res Social Adm Pharm. 2011 Dec;7(4):359-68. doi: 10.1016/j.sapharm.2010.10.007. Epub 2011 May 6. Res Social Adm Pharm. 2011. PMID: 21530417
-
Identifying high risk medications causing potential drug-drug interactions in outpatients: A prescription database study based on an online surveillance system.Res Social Adm Pharm. 2016 Jul-Aug;12(4):559-68. doi: 10.1016/j.sapharm.2015.09.004. Epub 2015 Sep 25. Res Social Adm Pharm. 2016. PMID: 26459026
-
Clinically significant drug-drug interactions between oral anticancer agents and nonanticancer agents: a Delphi survey of oncology pharmacists.Clin Ther. 2009;31 Pt 2:2379-86. doi: 10.1016/j.clinthera.2009.11.008. Clin Ther. 2009. PMID: 20110047
-
An evidence-based assessment of the clinical significance of drug-drug interactions between disease-modifying antirheumatic drugs and non-antirheumatic drugs according to rheumatologists and pharmacists.Clin Ther. 2009 Aug;31(8):1737-46. doi: 10.1016/j.clinthera.2009.08.009. Clin Ther. 2009. PMID: 19808132 Review.
Cited by
-
Current Knowledge about Providing Drug-Drug Interaction Services for Patients-A Scoping Review.Pharmacy (Basel). 2021 Mar 24;9(2):69. doi: 10.3390/pharmacy9020069. Pharmacy (Basel). 2021. PMID: 33805205 Free PMC article.
-
Medication safety in patients with hepatic impairment: A survey of community pharmacists' knowledge level and their practice in caring for these patients.Br J Clin Pharmacol. 2020 Apr;86(4):763-770. doi: 10.1111/bcp.14177. Epub 2020 Feb 3. Br J Clin Pharmacol. 2020. PMID: 31756269 Free PMC article.
-
Communicating tailored risk information of cancer treatment side effects: Only words or also numbers?BMC Med Inform Decis Mak. 2020 Oct 27;20(1):277. doi: 10.1186/s12911-020-01296-7. BMC Med Inform Decis Mak. 2020. PMID: 33109175 Free PMC article.
-
Respondent Understanding in Discrete Choice Experiments: A Scoping Review.Patient. 2021 Jan;14(1):17-53. doi: 10.1007/s40271-020-00467-y. Epub 2020 Nov 3. Patient. 2021. PMID: 33141359 Free PMC article.
-
Need for numbers: assessing cancer survivors' needs for personalized and generic statistical information.BMC Med Inform Decis Mak. 2022 Oct 5;22(1):260. doi: 10.1186/s12911-022-02005-2. BMC Med Inform Decis Mak. 2022. PMID: 36199092 Free PMC article.
References
-
- Schoemaker CG, van der Weijden T. Patiëntvoorkeur versus evidence based medicine: hadden de pioniers van EBM oog voor wat de patiënt wil? (Patient preferences versus evidence-based medicine: did the pioneers of evidence-based medicine take the patient’s preferences into account?) Ned Tijdschr Geneeskd. 2016;160:D24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

