Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells
- PMID: 28965766
- PMCID: PMC5755620
- DOI: 10.1016/j.stem.2017.08.014
Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells
Abstract
Lung alveoli, which are unique to air-breathing organisms, have been challenging to generate from pluripotent stem cells (PSCs) in part because there are limited model systems available to provide the necessary developmental roadmaps for in vitro differentiation. Here we report the generation of alveolar epithelial type 2 cells (AEC2s), the facultative progenitors of lung alveoli, from human PSCs. Using multicolored fluorescent reporter lines, we track and purify human SFTPC+ alveolar progenitors as they emerge from endodermal precursors in response to stimulation of Wnt and FGF signaling. Purified PSC-derived SFTPC+ cells form monolayered epithelial "alveolospheres" in 3D cultures without the need for mesenchymal support, exhibit self-renewal capacity, and display additional AEC2 functional capacities. Footprint-free CRISPR-based gene correction of PSCs derived from patients carrying a homozygous surfactant mutation (SFTPB121ins2) restores surfactant processing in AEC2s. Thus, PSC-derived AEC2s provide a platform for disease modeling and future functional regeneration of the distal lung.
Keywords: CRISPR; alveolar epithelial cell; development; disease modeling; embryonic stem cells; gene editing; human induced pluripotent stem cells; lung; surfactant; surfactant protein B.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
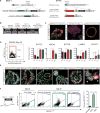

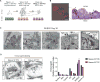
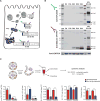


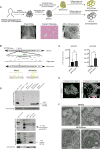
Comment in
-
The LUNGe to Model Alveolar Lung Diseases in a Dish.Cell Stem Cell. 2017 Oct 5;21(4):413-414. doi: 10.1016/j.stem.2017.09.009. Cell Stem Cell. 2017. PMID: 28985518
References
-
- Beers MF, Bates SR, Fisher AB. Differential extraction for the rapid purification of bovine surfactant protein B. Am J Physiol. 1992;262:L773–L778. - PubMed
-
- Beers MF, Kim CY, Dodia C, Fisher AB. Localization, synthesis, and processing of surfactant protein SP-C in rat lung analyzed by epitope-specific antipeptide antibodies. J Biol Chem. 1994;269:20318–20328. - PubMed
-
- Beers MF, Hamvas A, Moxley MA, Gonzales LW, Guttentag SH, Solarin KO, Longmore WJ, Nogee LM, Ballard PL. Pulmonary surfactant metabolism in infants lacking surfactant protein B. Am. J Respir Cell Mol Biol. 2000;22:380–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL136722/HL/NHLBI NIH HHS/United States
- U01 TR001810/TR/NCATS NIH HHS/United States
- R01 GM120060/GM/NIGMS NIH HHS/United States
- R24 HL123828/HL/NHLBI NIH HHS/United States
- TL1 TR001410/TR/NCATS NIH HHS/United States
- T32 HL007035/HL/NHLBI NIH HHS/United States
- U01 HL134745/HL/NHLBI NIH HHS/United States
- R01 HL059959/HL/NHLBI NIH HHS/United States
- K08 HL130582/HL/NHLBI NIH HHS/United States
- F31 HL129777/HL/NHLBI NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- L30 HL138777/HL/NHLBI NIH HHS/United States
- F31 HL134274/HL/NHLBI NIH HHS/United States
- I01 BX001176/BX/BLRD VA/United States
- R01 HL132999/HL/NHLBI NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- R01 HL128172/HL/NHLBI NIH HHS/United States
- R01 HL119436/HL/NHLBI NIH HHS/United States
- U01 HL099997/HL/NHLBI NIH HHS/United States
- R01 GM108807/GM/NIGMS NIH HHS/United States
- R01 HL095993/HL/NHLBI NIH HHS/United States
- R33 HL120760/HL/NHLBI NIH HHS/United States
- R56 HL059959/HL/NHLBI NIH HHS/United States
- U01 HL134766/HL/NHLBI NIH HHS/United States
- R01 HL122442/HL/NHLBI NIH HHS/United States
- R01 HL111449/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

