DNA damage mediates changes in neuronal sensitivity induced by the inflammatory mediators, MCP-1 and LPS, and can be reversed by enhancing the DNA repair function of APE1
- PMID: 28965839
- PMCID: PMC5954980
- DOI: 10.1016/j.neuroscience.2017.09.039
DNA damage mediates changes in neuronal sensitivity induced by the inflammatory mediators, MCP-1 and LPS, and can be reversed by enhancing the DNA repair function of APE1
Abstract
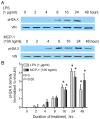
Although inflammation-induced peripheral sensitization oftentimes resolves as an injury heals, this sensitization can be pathologically maintained and contribute to chronic inflammatory pain. Numerous inflammatory mediators increase the production of reactive oxygen (ROS) and nitrogen species (RNS) during inflammation and in animal models of chronic neuropathic pain. Our previous studies demonstrate that ROS/RNS and subsequent DNA damage mediate changes in neuronal sensitivity induced by anticancer drugs and by ionizing radiation in sensory neurons, thus we investigated whether inflammation and inflammatory mediators also could cause DNA damage in sensory neurons and whether that DNA damage alters neuronal sensitivity. DNA damage was assessed by pH2A.X expression and the release of the neuropeptide, calcitonin gene-related peptide (CGRP), was measured as an index of neuronal sensitivity. Peripheral inflammation or exposure of cultured sensory neurons to the inflammatory mediators, LPS and MCP-1, elicited DNA damage. Moreover, exposure of sensory neuronal cultures to LPS or MCP-1 resulted in changes in the stimulated release of CGRP, without altering resting release or CGRP content. Genetically enhancing the expression of the DNA repair enzyme, apurinic/apyrimidinic endonuclease (APE1) or treatment with a small-molecule modulator of APE1 DNA repair activity, both which enhance DNA repair, attenuated DNA damage and the changes in neuronal sensitivity elicited by LPS or MCP-1. In conclusion, our studies demonstrate that inflammation or exposure to inflammatory mediators elicits DNA damage in sensory neurons. By enhancing DNA repair, we demonstrate that this DNA damage mediates the alteration of neuronal function induced by inflammatory mediators in peptidergic sensory neurons.
Keywords: DNA damage; E3330; TRPV1; apurinic/apyrimidinic endonuclease 1/redox effector factor 1; dorsal root ganglia; inflammation.
Copyright © 2017. Published by Elsevier Ltd.
Conflict of interest statement
Figures




Similar articles
-
The repair function of the multifunctional DNA repair/redox protein APE1 is neuroprotective after ionizing radiation.DNA Repair (Amst). 2011 Sep 5;10(9):942-52. doi: 10.1016/j.dnarep.2011.06.004. Epub 2011 Jul 8. DNA Repair (Amst). 2011. PMID: 21741887 Free PMC article.
-
Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons.Cancer Res. 2008 Aug 1;68(15):6425-34. doi: 10.1158/0008-5472.CAN-08-1173. Cancer Res. 2008. PMID: 18676868 Free PMC article.
-
Small molecule activation of apurinic/apyrimidinic endonuclease 1 reduces DNA damage induced by cisplatin in cultured sensory neurons.DNA Repair (Amst). 2016 May;41:32-41. doi: 10.1016/j.dnarep.2016.03.009. Epub 2016 Mar 29. DNA Repair (Amst). 2016. PMID: 27078577 Free PMC article.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
-
AP Endonuclease 1 as a Key Enzyme in Repair of Apurinic/Apyrimidinic Sites.Biochemistry (Mosc). 2016 Sep;81(9):951-67. doi: 10.1134/S0006297916090042. Biochemistry (Mosc). 2016. PMID: 27682167 Review.
Cited by
-
Syntaxin1A overexpression and pain insensitivity in individuals with 7q11.23 duplication syndrome.JCI Insight. 2024 Feb 22;9(4):e176147. doi: 10.1172/jci.insight.176147. JCI Insight. 2024. PMID: 38261410 Free PMC article.
-
Reflux conditions induce E-cadherin cleavage and EMT via APE1 redox function in oesophageal adenocarcinoma.Gut. 2023 Dec 7;73(1):47-62. doi: 10.1136/gutjnl-2023-329455. Gut. 2023. PMID: 37734913 Free PMC article.
-
APE1 Upregulates MMP-14 via Redox-Sensitive ARF6-Mediated Recycling to Promote Cell Invasion of Esophageal Adenocarcinoma.Cancer Res. 2019 Sep 1;79(17):4426-4438. doi: 10.1158/0008-5472.CAN-19-0237. Epub 2019 Jul 15. Cancer Res. 2019. PMID: 31308045 Free PMC article.
-
Inhibition of APE1/Ref-1 Redox Signaling Alleviates Intestinal Dysfunction and Damage to Myenteric Neurons in a Mouse Model of Spontaneous Chronic Colitis.Inflamm Bowel Dis. 2021 Feb 16;27(3):388-406. doi: 10.1093/ibd/izaa161. Inflamm Bowel Dis. 2021. PMID: 32618996 Free PMC article.
-
HDAC inhibitors as a potential therapy for chemotherapy-induced neuropathic pain.Inflammopharmacology. 2024 Aug;32(4):2153-2175. doi: 10.1007/s10787-024-01488-x. Epub 2024 May 18. Inflammopharmacology. 2024. PMID: 38761314 Review.
References
-
- Babior BM. Phagocytes and oxidative stress. The American journal of medicine. 2000;109:33–44. - PubMed
-
- Barzilai A, Biton S, Shiloh Y. The role of the DNA damage response in neuronal development, organization and maintenance. DNA repair. 2008;7:1010–1027. - PubMed
-
- Bauerova K, Bezek A. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. General physiology and biophysics. 1999:15–20. 18 Spec No. - PubMed
-
- Blum E, Procacci P, Conte V, Hanani M. Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience. 2014;274:209–217. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

