Hypomorphic Recessive Variants in SUFU Impair the Sonic Hedgehog Pathway and Cause Joubert Syndrome with Cranio-facial and Skeletal Defects
- PMID: 28965847
- PMCID: PMC5630196
- DOI: 10.1016/j.ajhg.2017.08.017
Hypomorphic Recessive Variants in SUFU Impair the Sonic Hedgehog Pathway and Cause Joubert Syndrome with Cranio-facial and Skeletal Defects
Abstract
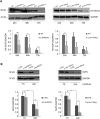
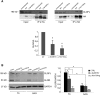
The Sonic Hedgehog (SHH) pathway is a key signaling pathway orchestrating embryonic development, mainly of the CNS and limbs. In vertebrates, SHH signaling is mediated by the primary cilium, and genetic defects affecting either SHH pathway members or ciliary proteins cause a spectrum of developmental disorders. SUFU is the main negative regulator of the SHH pathway and is essential during development. Indeed, Sufu knock-out is lethal in mice, and recessive pathogenic variants of this gene have never been reported in humans. Through whole-exome sequencing in subjects with Joubert syndrome, we identified four children from two unrelated families carrying homozygous missense variants in SUFU. The children presented congenital ataxia and cerebellar vermis hypoplasia with elongated superior cerebellar peduncles (mild "molar tooth sign"), typical cranio-facial dysmorphisms (hypertelorism, depressed nasal bridge, frontal bossing), and postaxial polydactyly. Two siblings also showed polymicrogyria. Molecular dynamics simulation predicted random movements of the mutated residues, with loss of the native enveloping movement of the binding site around its ligand GLI3. Functional studies on cellular models and fibroblasts showed that both variants significantly reduced SUFU stability and its capacity to bind GLI3 and promote its cleavage into the repressor form GLI3R. In turn, this impaired SUFU-mediated repression of the SHH pathway, as shown by altered expression levels of several target genes. We demonstrate that germline hypomorphic variants of SUFU cause deregulation of SHH signaling, resulting in recessive developmental defects of the CNS and limbs which share features with both SHH-related disorders and ciliopathies.
Keywords: GLI3; Joubert syndrome; SUFU; Sonic Hedgehog; ciliopathies; congenital ataxia; developmental defects; hypomorphic variants; molar tooth sign; polymicrogyria.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Gupta S., Sen J. Roof plate mediated morphogenesis of the forebrain: New players join the game. Dev. Biol. 2016;413:145–152. - PubMed
-
- Wechsler-Reya R.J., Scott M.P. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. - PubMed
-
- Lopez-Rios J. The many lives of SHH in limb development and evolution. Semin. Cell Dev. Biol. 2016;49:116–124. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

