Cell polarity and cytoskeletons-Lesson from the testis
- PMID: 28965865
- PMCID: PMC5889362
- DOI: 10.1016/j.semcdb.2017.09.037
Cell polarity and cytoskeletons-Lesson from the testis
Abstract
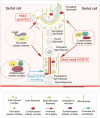
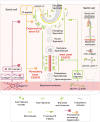
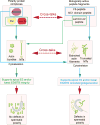
Cell polarity in the adult mammalian testis refers to the polarized alignment of developing spermatids during spermiogenesis and the polarized organization of organelles (e.g., phagosomes, endocytic vesicles, Sertoli cell nuclei, Golgi apparatus) in Sertoli cells and germ cells to support spermatogenesis. Without these distinctive features of cell polarity in the seminiferous epithelium, it is not possible to support the daily production of millions of sperm in the limited space provided by the seminiferous tubules in either rodent or human males through the adulthood. In short, cell polarity provides a novel mean to align spermatids and the supporting organelles (e.g., phagosomes, Golgi apparatus, endocytic vesicles) in a highly organized fashion spatially in the seminiferous epithelium during the epithelial cycle of spermatogenesis. This is analogous to different assembling units in a manufacturing plant such that as developing spermatids move along the "assembly line" conferred by Sertoli cells, different structural/functional components can be added to (or removed from) the developing spermatids during spermiogenesis, so that functional spermatozoa are produced at the end of the assembly line. Herein, we briefly review findings regarding the regulation of cell polarity in the testis with specific emphasis on developing spermatids, supported by an intriguing network of regulatory proteins along a local functional axis. Emerging evidence has suggested that cell cytoskeletons provide the tracks which in turn confer the unique assembly lines in the seminiferous epithelium. We also provide some thought-provoking concepts based on which functional experiments can be designed in future studies.
Keywords: Actin; Actin nucleation; Blood-testis barrier; Cell polarity; Cytoskeleton; Ectoplasmic specialization; Microtubules; Seminiferous epithelial cycle; Sertoli cells; Spermatids; Spermatogenesis; Testis.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Regulation of spermatid polarity by the actin- and microtubule (MT)-based cytoskeletons.Semin Cell Dev Biol. 2018 Sep;81:88-96. doi: 10.1016/j.semcdb.2018.01.013. Epub 2018 Jul 12. Semin Cell Dev Biol. 2018. PMID: 29410206 Free PMC article. Review.
-
Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis.Am J Physiol Endocrinol Metab. 2014 Nov 1;307(9):E738-53. doi: 10.1152/ajpendo.00113.2014. Epub 2014 Aug 26. Am J Physiol Endocrinol Metab. 2014. PMID: 25159326 Free PMC article.
-
Planar cell polarity protein Dishevelled 3 (Dvl3) regulates ectoplasmic specialization (ES) dynamics in the testis through changes in cytoskeletal organization.Cell Death Dis. 2019 Feb 26;10(3):194. doi: 10.1038/s41419-019-1394-7. Cell Death Dis. 2019. PMID: 30808893 Free PMC article.
-
Cell polarity and planar cell polarity (PCP) in spermatogenesis.Semin Cell Dev Biol. 2018 Sep;81:71-77. doi: 10.1016/j.semcdb.2017.09.008. Epub 2017 Sep 29. Semin Cell Dev Biol. 2018. PMID: 28923514 Free PMC article. Review.
-
Coordination of Actin- and Microtubule-Based Cytoskeletons Supports Transport of Spermatids and Residual Bodies/Phagosomes During Spermatogenesis in the Rat Testis.Endocrinology. 2016 Apr;157(4):1644-59. doi: 10.1210/en.2015-1962. Epub 2016 Feb 19. Endocrinology. 2016. PMID: 26894662 Free PMC article.
Cited by
-
Preliminary Investigation on the Involvement of Cytoskeleton-Related Proteins, DAAM1 and PREP, in Human Testicular Disorders.Int J Mol Sci. 2021 Jul 28;22(15):8094. doi: 10.3390/ijms22158094. Int J Mol Sci. 2021. PMID: 34360857 Free PMC article.
-
BCL2-associated athanogene 6 exon24 contributes to testosterone synthesis and male fertility in mammals.Cell Prolif. 2022 Jul;55(7):e13281. doi: 10.1111/cpr.13281. Epub 2022 Jun 10. Cell Prolif. 2022. PMID: 35688694 Free PMC article.
-
HUWE1 is involved in Sertoli cell polarity establishment by ubiquitination mediated degradation of WT1.Cell Mol Life Sci. 2025 Jul 3;82(1):271. doi: 10.1007/s00018-025-05779-6. Cell Mol Life Sci. 2025. PMID: 40610632 Free PMC article.
-
Loss of myosin VI expression affects acrosome/acroplaxome complex morphology during mouse spermiogenesis†.Biol Reprod. 2020 Aug 21;103(3):521-533. doi: 10.1093/biolre/ioaa071. Biol Reprod. 2020. PMID: 32412041 Free PMC article.
-
Regulation of spermatid polarity by the actin- and microtubule (MT)-based cytoskeletons.Semin Cell Dev Biol. 2018 Sep;81:88-96. doi: 10.1016/j.semcdb.2018.01.013. Epub 2018 Jul 12. Semin Cell Dev Biol. 2018. PMID: 29410206 Free PMC article. Review.
References
-
- de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 1. Raven Press; New York: 1988. pp. 837–932.
-
- Cheng CY, Mruk DD. Sertoli Cell Biology. 2. Griswold, M.D.: Amsterdam, Elsevier; 2015. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium; pp. 333–383. - DOI
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–278. - PubMed
-
- Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci. 1987;513:1–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

