Coordinated Splicing of Regulatory Detained Introns within Oncogenic Transcripts Creates an Exploitable Vulnerability in Malignant Glioma
- PMID: 28966034
- PMCID: PMC5929990
- DOI: 10.1016/j.ccell.2017.08.018
Coordinated Splicing of Regulatory Detained Introns within Oncogenic Transcripts Creates an Exploitable Vulnerability in Malignant Glioma
Abstract
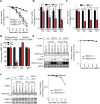
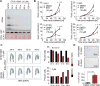
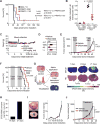
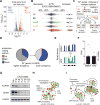
Glioblastoma (GBM) is a devastating malignancy with few therapeutic options. We identify PRMT5 in an in vivo GBM shRNA screen and show that PRMT5 knockdown or inhibition potently suppresses in vivo GBM tumors, including patient-derived xenografts. Pathway analysis implicates splicing in cellular PRMT5 dependency, and we identify a biomarker that predicts sensitivity to PRMT5 inhibition. We find that PRMT5 deficiency primarily disrupts the removal of detained introns (DIs). This impaired DI splicing affects proliferation genes, whose downregulation coincides with cell cycle defects, senescence and/or apoptosis. We further show that DI programs are evolutionarily conserved and operate during neurogenesis, suggesting that they represent a physiological regulatory mechanism. Collectively, these findings reveal a PRMT5-regulated DI-splicing program as an exploitable cancer vulnerability.
Keywords: CLNS1A; EPZ015666; GBM; PRMT5; RIOK1; biomarker; splicing addiction.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Exploiting the Hidden Treasure of Detained Introns.Cancer Cell. 2017 Oct 9;32(4):393-395. doi: 10.1016/j.ccell.2017.09.005. Cancer Cell. 2017. PMID: 29017049 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

