Misreporting of contraceptive hormone use in clinical research participants
- PMID: 28966052
- PMCID: PMC5858917
- DOI: 10.1016/j.contraception.2017.09.013
Misreporting of contraceptive hormone use in clinical research participants
Abstract
Objective: Researchers traditionally rely on participant self-report for contraceptive use. We hypothesized that self-reported contraceptive use by clinical research participants may disagree with objectively measured hormonal status.
Study design: We enrolled women in Harare, Zimbabwe, aged 18-34, who by self-report had not used hormonal or intrauterine contraception for >30 days, or depot medroxyprogesterone acetate for >10 months, into a study designed to assess biologic changes with contraceptive initiation and use. Blood samples obtained at enrollment and each follow-up visit (N=1630 from 447 participants) were evaluated by mass spectrometry for exogenous hormones. We individually interviewed a subset of participants (n=20) with discrepant self-reported and measured serum hormones to better understand nondisclosure of contraceptive use.
Results: Discrepant with self-reported nonuse of hormonal contraception, synthetic progestogens were detectable in 120/447 (27%, 95% confidence interval 23%-31%) enrolled women. Measured exogenous hormones consistent with use of contraceptive pills (n=102), injectables (n=20) and implants (n=3) were detected at enrollment, with 7 women likely using >1 contraceptive. In-depth interviews revealed that participants understood the requirement to be hormone free at enrollment (100%). Most (85%) cited partner noncooperation with condoms/withdrawal and/or pregnancy concerns as major reasons for nondisclosed contraceptive use. All interviewed women (100%) cited access to health care as a primary motivation for study participation. Of participants who accurately reported nonuse of hormonal contraception at enrollment, 41/327 (12.5%) had objective evidence of nonstudy progestin use at follow-up that disagreed with self-reported nonuse.
Conclusions: Women joining contraceptive research studies may misrepresent their use of nonstudy contraceptive hormones at baseline and follow-up. Objective measures of hormone use are needed to ensure that study population exposures are accurately categorized.
Implications statement: Among Zimbabwean women participating in a contraceptive research study, 27% had objective evidence of use of nonstudy contraceptives at enrollment that disagreed with self-report. Studies that rely on self-report to identify contraceptive hormone exposure could suffer from significant misclassification.
Keywords: Hormonal contraception; LARC; Misreporting; Oral contraceptive pills; Self-report.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
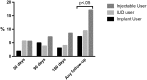
References
-
- Kourtis A.P., Haddad L., Tang J., Chinula L., Hurst S., Wiener J. A randomized clinical trial on the effects of progestin contraception in the genital tract of HIV-infected and uninfected women in Lilongwe, Malawi: addressing evolving research priorities. Contemp Clin Trials. 2017;52:27–34. - PMC - PubMed
-
- Byrne E.H., Anahtar M.N., Cohen K.E., Moodley A., Padavattan N., Ismail N. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: a prospective cohort study. Lancet Infect Dis. 2016;16:441–448. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

