A Potent Germline-like Human Monoclonal Antibody Targets a pH-Sensitive Epitope on H7N9 Influenza Hemagglutinin
- PMID: 28966056
- PMCID: PMC6290738
- DOI: 10.1016/j.chom.2017.08.011
A Potent Germline-like Human Monoclonal Antibody Targets a pH-Sensitive Epitope on H7N9 Influenza Hemagglutinin
Abstract
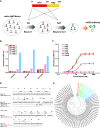
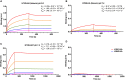
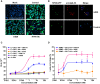
The H7N9 influenza virus causes high-mortality disease in humans but no effective therapeutics are available. Here we report a human monoclonal antibody, m826, that binds to H7 hemagglutinin (HA) and protects against H7N9 infection. m826 binds to H7N9 HA with subnanomolar affinity at acidic pH and 10-fold lower affinity at neutral pH. The high-resolution (1.9 Å) crystal structure of m826 complexed with H7N9 HA indicates that m826 binds an epitope that may be fully exposed upon pH-induced conformational changes in HA. m826 fully protects mice against lethal challenge with H7N9 virus through mechanisms likely involving antibody-dependent cell-mediated cytotoxicity. Interestingly, immunogenetic analysis indicates that m826 is a germline antibody, and m826-like sequences can be identified in H7N9-infected patients, healthy adults, and newborn babies. These m826 properties offer a template for H7N9 vaccine immunogens, a promising candidate therapeutic, and a tool for exploring mechanisms of virus infection inhibition by antibodies.
Keywords: ADCC; H7N9 influenza; germline; hemagglutinin; monoclonal antibody.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Identification of H7N9 hemagglutinin novel protein epitopes that elicit strong antibody-dependent, cell-mediated cytotoxic activities with protection from influenza infection in mouse model.Cell Immunol. 2021 Jan;359:104255. doi: 10.1016/j.cellimm.2020.104255. Epub 2020 Dec 6. Cell Immunol. 2021. PMID: 33316647
-
A Replication-Defective Influenza Virus Harboring H5 and H7 Hemagglutinins Provides Protection against H5N1 and H7N9 Infection in Mice.J Virol. 2021 Jan 13;95(3):e02154-20. doi: 10.1128/JVI.02154-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177192 Free PMC article.
-
Both Neutralizing and Non-Neutralizing Human H7N9 Influenza Vaccine-Induced Monoclonal Antibodies Confer Protection.Cell Host Microbe. 2016 Jun 8;19(6):800-13. doi: 10.1016/j.chom.2016.05.014. Cell Host Microbe. 2016. PMID: 27281570 Free PMC article.
-
Cross-conservation of T-cell epitopes: now even more relevant to (H7N9) influenza vaccine design.Hum Vaccin Immunother. 2014;10(2):256-62. doi: 10.4161/hv.28135. Epub 2014 Feb 13. Hum Vaccin Immunother. 2014. PMID: 24525618 Free PMC article. Review.
-
T cell epitope engineering: an avian H7N9 influenza vaccine strategy for pandemic preparedness and response.Hum Vaccin Immunother. 2018;14(9):2203-2207. doi: 10.1080/21645515.2018.1495303. Epub 2018 Sep 5. Hum Vaccin Immunother. 2018. PMID: 30015562 Free PMC article. Review.
Cited by
-
Potent germline-like monoclonal antibodies: rapid identification of promising candidates for antibody-based antiviral therapy.Antib Ther. 2021 May 17;4(2):89-98. doi: 10.1093/abt/tbab008. eCollection 2021 Apr. Antib Ther. 2021. PMID: 34104872 Free PMC article.
-
Ultrapotent Human Neutralizing Antibody Repertoires Against Middle East Respiratory Syndrome Coronavirus From a Recovered Patient.J Infect Dis. 2018 Sep 8;218(8):1249-1260. doi: 10.1093/infdis/jiy311. J Infect Dis. 2018. PMID: 29846635 Free PMC article.
-
Generation of neutralizing and non-neutralizing monoclonal antibodies against H7N9 influenza virus.Emerg Microbes Infect. 2020 Mar 20;9(1):664-675. doi: 10.1080/22221751.2020.1742076. eCollection 2020. Emerg Microbes Infect. 2020. PMID: 32193996 Free PMC article.
-
Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29832-29838. doi: 10.1073/pnas.2010197117. Epub 2020 Nov 2. Proc Natl Acad Sci U S A. 2020. PMID: 33139569 Free PMC article.
-
A protective and broadly binding antibody class engages the influenza virus hemagglutinin head at its stem interface.bioRxiv [Preprint]. 2024 Jul 1:2023.12.13.571543. doi: 10.1101/2023.12.13.571543. bioRxiv. 2024. Update in: mBio. 2025 Jun 11;16(6):e0089225. doi: 10.1128/mbio.00892-25. PMID: 38168412 Free PMC article. Updated. Preprint.
References
-
- Agrawal A.S., Ying T., Tao X., Garron T., Algaissi A., Wang Y., Wang L., Peng B.H., Jiang S., Dimitrov D.S. Passive transfer of A germline-like neutralizing human monoclonal antibody protects transgenic mice against lethal Middle East respiratory syndrome coronavirus infection. Sci. Rep. 2016;6:31629. - PMC - PubMed
-
- Bernasconi-Elias P., Hu T., Jenkins D., Firestone B., Gans S., Kurth E., Capodieci P., Deplazes-Lauber J., Petropoulos K., Thiel P. Characterization of activating mutations of NOTCH3 in T-cell acute lymphoblastic leukemia and anti-leukemic activity of NOTCH3 inhibitory antibodies. Oncogene. 2016;35:6077–6086. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

