Actin Turnover in Lamellipodial Fragments
- PMID: 28966086
- PMCID: PMC5679493
- DOI: 10.1016/j.cub.2017.08.066
Actin Turnover in Lamellipodial Fragments
Abstract
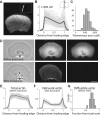
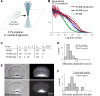
Actin turnover is the central driving force underlying lamellipodial motility. The molecular components involved are largely known, and their properties have been studied extensively in vitro. However, a comprehensive picture of actin turnover in vivo is still missing. We focus on fragments from fish epithelial keratocytes, which are essentially stand-alone motile lamellipodia. The geometric simplicity of the fragments and the absence of additional actin structures allow us to characterize the spatiotemporal lamellipodial actin organization with unprecedented detail. We use fluorescence recovery after photobleaching, fluorescence correlation spectroscopy, and extraction experiments to show that about two-thirds of the lamellipodial actin diffuses in the cytoplasm with nearly uniform density, whereas the rest forms the treadmilling polymer network. Roughly a quarter of the diffusible actin pool is in filamentous form as diffusing oligomers, indicating that severing and debranching are important steps in the disassembly process generating oligomers as intermediates. The remaining diffusible actin concentration is orders of magnitude higher than the in vitro actin monomer concentration required to support the observed polymerization rates, implying that the majority of monomers are transiently kept in a non-polymerizable "reserve" pool. The actin network disassembles and reassembles throughout the lamellipodium within seconds, so the lamellipodial network turnover is local. The diffusible actin transport, on the other hand, is global: actin subunits typically diffuse across the entire lamellipodium before reassembling into the network. This combination of local network turnover and global transport of dissociated subunits through the cytoplasm makes actin transport robust yet rapidly adaptable and amenable to regulation.
Keywords: actin turnover; biophysical modeling; cell motility; fish keratocytes.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
-
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiological reviews. 2014;94:235–263. - PubMed
-
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

